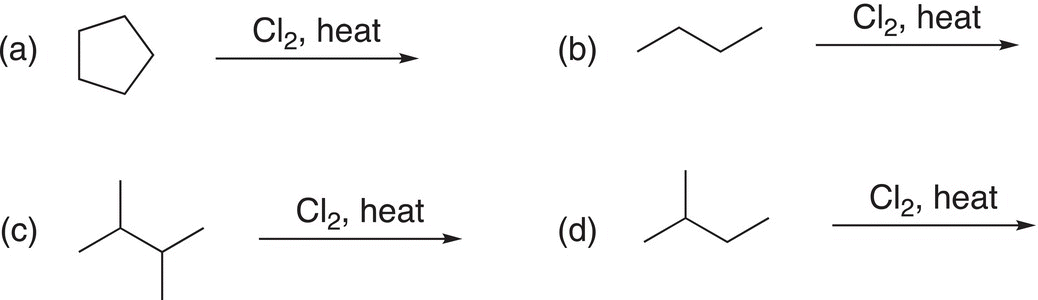Organic Chemistry: Concepts and Applications - Headley Allan D. 2020
Free Radical Substitution Reactions Involving Alkanes
14.3 Chlorination of Alkanes
Alkanes are fairly inert compounds; some alkanes are used as solvents to provide inert media for different reactions that we will see in later chapters. In addition to combustion, alkanes undergo another type of reaction that is very important to organic chemists, and that is the reaction with halogens, specifically chlorine or bromine in the presence of energy in the form of heat or light. As the name suggests, this reaction involves the reaction of alkanes and bromine or chlorine and is called bromination or chlorination of alkanes, respectively. These reactions are performed in the presence of light or heat and are described as substitution reactions since a hydrogen or more than one hydrogen atoms of an alkane reactant are substituted for a halogen or more than one halogen in the product. Most of the reactions that will be encountered in this chapter involve the substitution of one hydrogen in the alkane for a halogen in the product.
The products produced upon the chlorination of alkanes are alkyl halides, most are important industrial raw material for the synthesis of numerous other chemicals. For example, chloromethane was used once as a refrigerant, but discontinued owing to its flammability and toxicity. Today, it is widely used as a chemical intermediate for the production of different compounds, including polymers. Other chloroalkanes are widely used as a solvent in the research labs and in the production of rubber and in the petroleum refining industry. The chlorination of methane is shown in Reaction (14-5).
(14-5)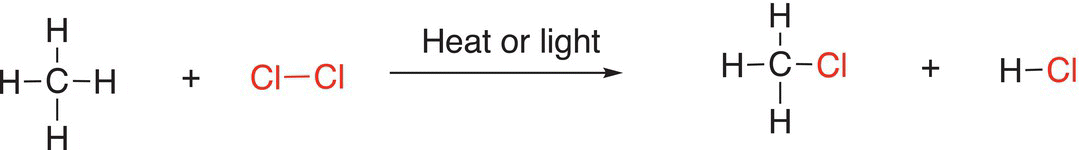
To be able to predict the products of these and other similar reactions, a thorough understanding of how the reaction occurs is essential. Before we examine the reaction mechanism for the chlorination of alkanes, let us examine the process of bond reorganization for the reaction shown in Reaction (14-5). It should be obvious that this reaction is a substitution reaction in which a Cl─Cl bond has to be broken and the chlorine atoms form two new bonds, a new H─Cl bond and a new C─Cl bond in the CH3Cl product. This also means that a C─H bond had to be broken in the reactant molecule in order for these new bonds to be formed in the products.
14.3.1 Mechanism for the Chlorination of Methane
Now that we have an idea of what has to occur for a chlorination substitution reaction to take place, let us now try to put that concept in the form of a reaction mechanism. To better understand how the reaction shown in 14-5 occurs, imagine a reaction vessel, which contains methane and chlorine. These two compounds will exist in this vessel until they are exposed to heat or light. Under either of these reaction conditions, the energy will break the weakest bond of either of these two reactant molecules. The bond dissociation energy for the C─H bond of methane is 105 kcal mol−1, while the bond dissociation energy for the Cl─Cl bond is 58 kcal mol−1 as shown in Reactions (14-6) and (14-7).
(14-6)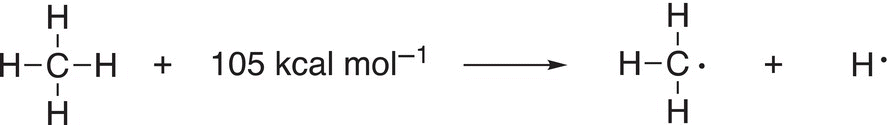
(14-7)![]()
Stronger bonds, such as the carbon—hydrogen bonds of methane, are not readily broken in the presence of heat or light. Thus, by introducing energy in the reaction vessel, which contains chlorine and methane, a homolytic cleavage of the Cl─Cl bond will occur, rather than the C─H bond as shown in Reaction (14-8). Note again that a single-barbed arrow is used to indicate the movement of one electron and to demonstrate how bond cleavage occurs.
(14-8)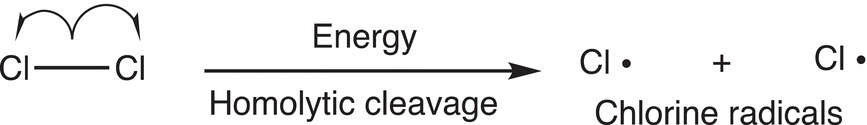
This initial step of this reaction mechanism is also known as the initiation step. In this step, free radicals (chlorine radicals) are generated. A free radical is an atom or group of atoms that has an unpaired electron present. Free radicals are intermediates that are typically generated throughout the course of some reaction and most are fairly reactive. Once a fairly reactive radical intermediate is generated, it will react in order to gain another odd electron, usually to form a covalent bond. Since the chlorine radical is a very reactive intermediate, it will react with methane in an effort to become neutral. This reaction can be accomplished by the abstraction of a hydrogen atom (a hydrogen radical) to form HCl. However, a newly formed radical is also formed in this process as shown below, in this case, a methyl radical as shown in Reaction (14-9).
(14-9)
This organic-free radical intermediate that is formed is also very reactive, and this methyl radical will react with unreacted chlorine molecules by the abstraction of a chlorine atom (a chlorine radical) to form CH3Cl and a chlorine atom, as shown in Reaction (14-10).
(14-10)
The steps of the reaction mechanism in which radicals are generated are called the propagation steps. As you can see the intermediate methyl radical eventually goes on to form one of the final products as shown in Reaction (14-10). Another way in which radicals can gain another electron to form a covalent bond is for two radicals to combine to form a neutral molecule. If a methyl radical and a chlorine radical come into contact with each other, they will form a neutral product, chloromethane, as shown in Reaction (14-11).
(14-11)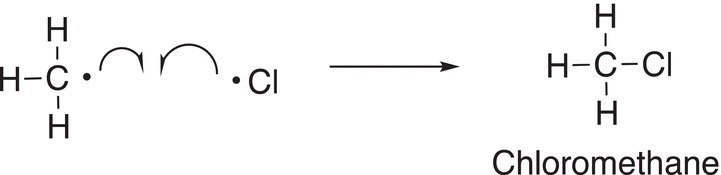
This step of the mechanism is called a termination step since radicals are converted into neutral products. As you can imagine, this is not the only termination step possible since there are other radicals that are present in the reaction vessel. Another termination reaction that is possible is shown in Reaction (14-12).
(14-12)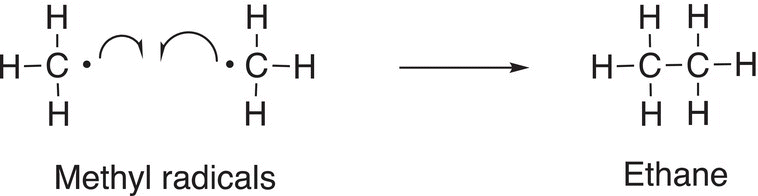
As you can imagine from the mechanism described above, there are other possible products. For example, the chloromethyl radical could be generated during a propagation step of the reaction mechanism, as shown in Reaction (14-13).
(14-13)
It is possible for this chloromethane radical to react with another chlorine atom or even with another chloromethyl radical to produce different products as shown in Reactions (14-14) and (14-15).
(14-14)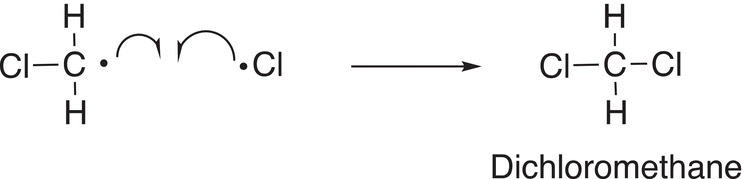
(14-15)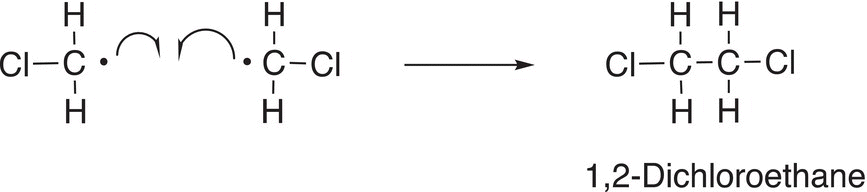
Depending on the reaction conditions, it is possible to target one of these products as the major organic product. A high concentration of chlorine will result in various multi-chlorination products. On the other hand, a high concentration of methane will result in chloromethane as the major organic product. For our discussion, we will assume that these substitution reactions give the mono-chlorinated product as the major organic product.
14.3.2 Chlorination of Other Alkanes
Let us now look at the chlorination of ethane, the reaction is shown in Reaction (14-16).
(14-16)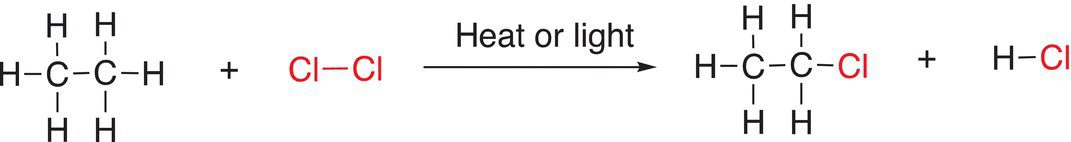
All six C─H bonds of ethane are equivalent, and they are all primary hydrogens. Thus, the mono-chlorination product shown in Reaction (14-16) is not surprising based on the mechanism described earlier. The chlorination of propane, however, gives two possible mono-chlorination products as shown in Reaction (14-17).
(14-17)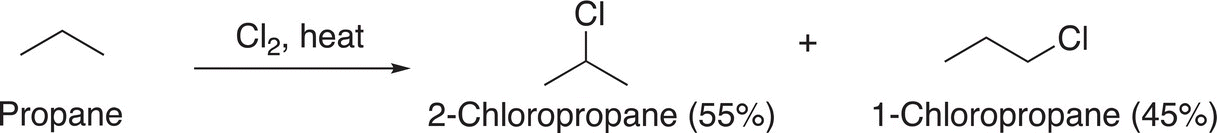
Note that there is a mixture of mono-chlorinated products, and they are formed in almost equal amounts. Thus, if one wanted to synthesize a large percentage of 2-chloropropane, only about 55% of the desired product would be obtained; the other product would be 1-chloropropane, an unwanted product. Let us take a closer look at the structures of methane, ethane and propane. All the C─H bonds of methane are equivalent and classified as methyl hydrogens. All the C─H bonds of ethane are equivalent and are described as primary hydrogens, but not all the C─H bonds of propane are equivalent. For propane, there are primary and secondary hydrogens and this difference in the type of hydrogen results in different products for the chlorination of propane, compared to methane and ethane. For molecules that have only one type of carbon—hydrogen bonds or hydrogens, only one mono-chlorination product will result, as shown in Reaction (14-18) for the chlorination of cyclohexane and Reaction (14-19) for the chlorination of 2,2-dimethylpropane.
(14-18)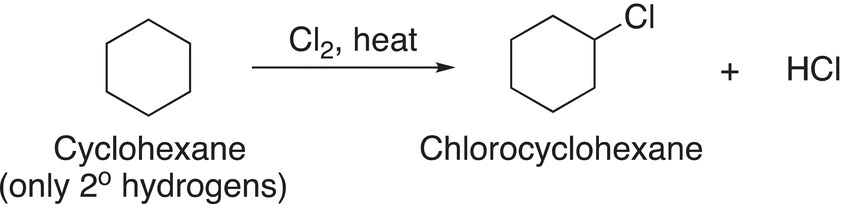
(14-19)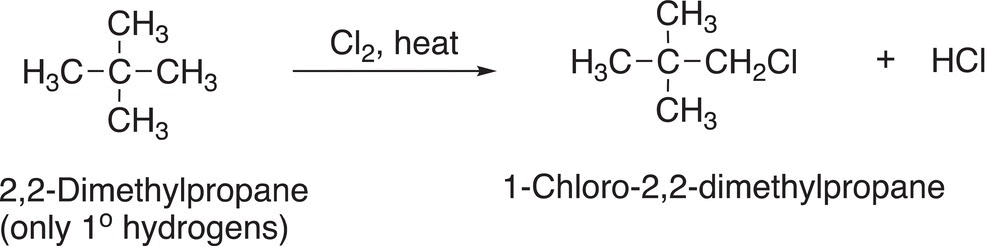
Thus, it is extremely important to be able to recognize different types of C─H bonds or hydrogens of alkanes in order to properly predict the organic products for these types of free radical substitution reactions.
Problem 14.4
i. Identify the different types of hydrogens of in the following molecules?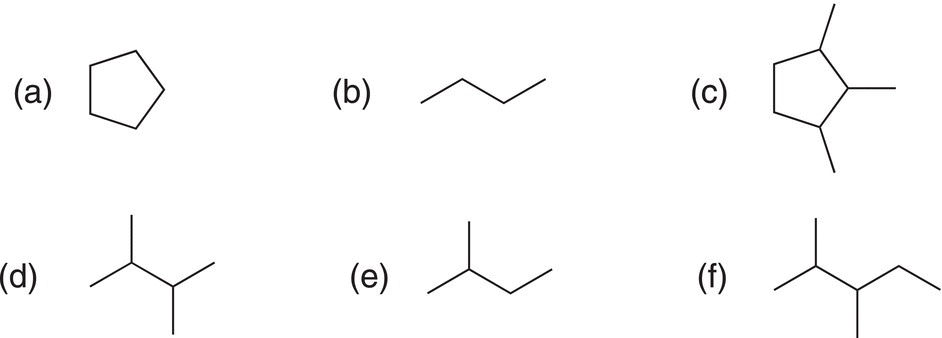
ii. Give the mechanism (initiation, propagation, and termination steps) for the reaction shown below.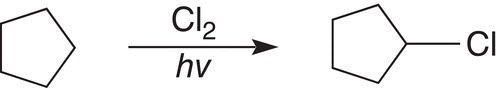
iii. Determine the chlorination products for the following reactions. Note that some may give more than one chlorination product.