Process Technology: An Introduction - Haan A.B. 2015
4 Chemical reactors and their industrial applications
4.6 Fixed and moving bed reactors
4.6.1 Fixed bed reactors
Catalytic fixed bed reactors are the most important types of reactor for the synthesis of large-scale base chemicals and intermediates. In these reactors the reaction takes place in the form of a heterogeneously catalyzed gas reaction on the surface of catalysts that are arranged as a so-called fixed bed in the reactor. In addition to synthesis of valuable chemicals, fixed bed reactors have been increasingly used to treat harmful and toxic substances. For example, the reaction chambers used to remove nitrogen oxides from power station flue gases constitute the largest type of fixed bed reactors regarding reactor volume and throughput. Automobile exhaust purification represents by far the most widely employed application of fixed bed reactors.
With regard to application and construction it is convenient to differentiate between fixed bed reactors for adiabatic and nonadiabatic operation. Adiabatic reactors are used only where the heat of reaction is small, or where there is only one major reaction pathway. In these cases no adverse effects on selectivity or yield due to adiabatic temperature development are expected. Reactions with a large heat of reaction as well as reactions that are extremely temperature sensitive are carried out in reactors with provisions for heat removal. The most common arrangement is the multitubular fixed bed reactor, in which the catalyst is arranged in tubes. The heat carrier is circulated externally outside the tube.
4.6.2 Adiabatic fixed bed reactors
Adiabatic fixed bed reactors are the oldest fixed bed reactor configurations. In the simplest case they consist of a cylindrical jacket in which the catalyst is loosely packed and traversed in the axial direction (Fig. 4.21). To avoid catalyst abrasion by partial fluidization, catalyst packings are always traversed from top to bottom. An alternative design is encountered in the oxidation of ammonia to nitrogen oxides, where extremely short residence times are required. This is achieved with a bed of large diameter and low height, followed by direct quenching of the reaction products (Fig. 4.22). The fixed bed consists of several layers of platinum wire gauze.
Purely adiabatic fixed bed reactors are used mainly for reactions with a small heat of reaction. Such reactions are primarily involved in gas purification, where small amounts of interfering components are converted to noninterfering compounds. The chambers used to remove NOx from power station flue gases, with catalyst volumes of more than 1000m3, are the largest adiabatic reactors. Exhaust catalysts for internal combustion engines, with a catalyst volume of 1 liter, are the smallest. Typical chemical applications include the methanation of CO and CO2 residues in ammonia synthesis gas and the hydrogenation of small amounts of unsaturated compounds in hydrocarbon streams.

Fig. 4.21: Adiabatic fixed bed reactor.
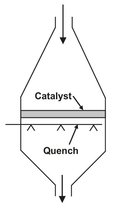
Fig. 4.22: Schematic of the ammonia oxidation reactor.
4.6.3 Fixed bed reactors with supply or removal of heat
In the majority of fixed bed reactors for industrial synthesis reactions, direct or indirect supply or removal of heat in the catalyst bed is utilized to adapt the temperature profile. Here a clear development trend can be observed. Starting with the adiabatic reactor, higher conversions were achieved at the same mean temperature level when several adiabatic stages were introduced with intermediate heating or cooling after each stage. The simplest form involves injecting hot or cold gas between the stages. The main disadvantage of this type of temperature control strategy are increasing cross-sectional loading from stage to stage, and the mixing of hot and cold streams, which is energetically unfavorable. A further development was the replacement of injection cooling by interstage heat exchangers (Fig. 4.23), through which the required or released heat of reaction is supplied or removed. The development of reactors in which the heat exchange surfaces are integrated in the fixed bed occurred parallel with the development of multistage adiabatic reactors with intermediate heating or cooling. The multitubular fixed bed reactor (Fig. 4.24) constitutes the oldest and still predominant representative of this class of fixed bed reactors, characterized by reaction tubes of 20-80 mm internal diameter. Here the catalyst packing is located in the individual tubes of the tube bundle. Depending on the reactor capacity the number of tubes varies between 30 and 30 000. The heat-transfer medium is circulated around the tube bundle and through an external heat exchanger.
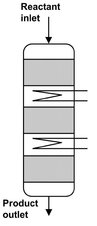
Fig. 4.23: Multistage fixed bed reactor.
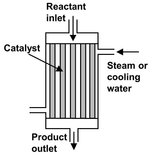
Fig. 4.24: Multitubular fixed bed reactor.
Adiabatic multistage fixed bed reactors with intermediate cooling or heating are nowadays used particularly where the reaction proceeds selectively to a single product but is limited by the thermodynamic equilibrium conditions. Intermediate cooling or heating shifts the reaction equilibrium to higher conversion. Typical examples comprise the synthesis of ammonia, sulfur trioxide, and methanol. In these exothermic reactions the equilibrium conversion decreases with increasing temperature. The kinetically optimum reaction pathway with the smallest required catalyst volumes is obtained by dividing the reactor into a large number of small stages. In practice, a balance must be found between equipment costs associated with a large number of stages and the savings in catalyst. Conventional multistage reactors for this class of reactions have therefore three to five stages. Fig. 4.25 shows the layout of an ammonia synthesis reactor designed on this basis. Reactor design for ammonia synthesis is subject to numerous constraints. Equilibrium favors high pressures (150-300 bar) and low temperatures (430-480 °C), while kinetics favor the reverse conditions. For structural reasons the heat exchanger is incorporated between the inflow and outflow in the lowest part of the pressure casing. The reaction gas then flows upward in the annular gap between the pressure casing and the fixed beds, whereby it is further heated, and at the same time protects the pressure-bearing structural components against excessively high fixed bed temperatures to prevent hydrogen embrittlement. The three adiabatic fixed beds are traversed from top to bottom. A part of the heat of reaction is utilized to generate steam in the two intermediate heat exchangers. To start up the cold reactor, hot gas must be added to the uppermost bed, for example through an external start-up preheater.
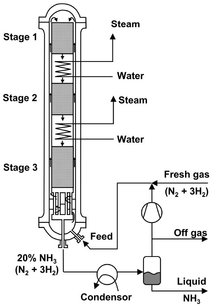
Fig. 4.25: Schematic of a multistage reactor for ammonia synthesis. Adapted from [10].
Multitubular fixed bed reactors are used for many highly exothermic reactions (oxidation, alkylation, hydrogenation) or highly endothermic reactions (steam reforming, dehydrogenation, dehydration). Some typical examples of these reaction systems involve the hydrogenation of phenol to cyclohexanone, the dehydrogenation of cyclohexanol to cyclohexanone, and the conversion of docan into α-picoline. The hydrogenation of phenol to cyclohexanone is normally carried out in the gas phase at 140-170 °C and atmospheric pressure with a noble-metal catalyst.
4.6.4 Moving bed reactors
Moving bed reactors operate as continuous plug flow reactors for solid products. They use gravity to transport the solids continuously through the reactor. Because the gas or liquid has to flow through the bed of solids, mass and heat transport between the phases is relatively good. One of the reasons for using a moving bed reactor is the long residence times that can be attained for the solids. This is for instance used in the process for Stanyl (Nylon 4,6) preparation (Fig. 4.26) from diaminobutane and adipic acid:
![]()
First a relatively low viscous aqueous prepolymer solution is produced in a stirred tank reactor at a temperature between 180 and 210 °C and pressure of 10-19 bar. After removing the excess of water by flash evaporation, the prepolymer is obtained as small particles with a size of 30-1000 μm. To avoid dust problems the prepolymer particles are pelletized and continuously introduced into a vertical cylinder, the so-called moving bed reactor. Hot nitrogen gas with a temperature of 240 °C is used to strip the residual water from the prepolymer pellets to force the equilibrium of the polycondensation reaction to the desired degree of polymerization. The produced stanyl polymer is continuously withdrawn from the reactor and cooled before packaging.
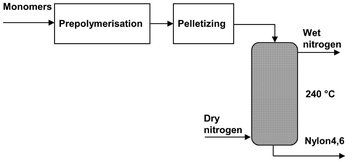
Fig. 4.26: Schematic of stanyl production with a moving bed reactor.