Process Technology: An Introduction - Haan A.B. 2015
4 Chemical reactors and their industrial applications
4.7 Fluidized bed reactors
The fluidization principle was first used on an industrial scale in 1922 for the gasification of fine-grained coal. Since then, fluidized beds have been applied in many industrially important processes. The present spectrum of applications extends from a number of physical processes such as cooling, heating, drying, sublimation, adsorption, coating, and granulation to many heterogeneous catalytic gas-phase reactions as well as noncatalytic reactions.
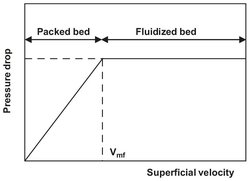
Fig. 4.27: Pressure drop as a function of the superficial velocity for a packed and fluidized bed.
4.7.1 The fluidization principle
A fluidized bed is created by the upward flow of gas or liquid through a bed of particles. If the superficial velocity of the fluid is gradually increased, a point is reached at which the bed becomes unstable. This is the point at which the pressure drop is sufficient to overcome the weight of the particle bed, the so-called minimum fluidization velocity vmf (Fig. 4.27). Increased velocities are then possible, until the point is reached at which complete particle entrainment occurs.
As the superficial velocity of the fluid increases beyond the minimum fluidization velocity, two things can happen. In fluidization with a liquid the bed begins to expand uniformly. The motion of the particles is random and localized. As the superficial velocity is increased, the particles move further apart to accommodate the flow and the bed expands further.
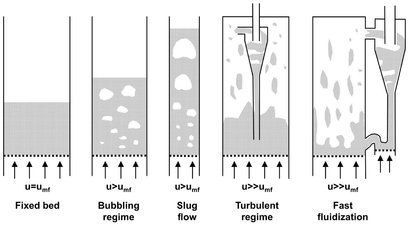
Fig. 4.28: Forms of gas-solids fluidized beds. Adapted from [49].
When a gas is used to fluidize the particles, virtually solids-free gas bubbles begin to form (Fig. 4.28). As the superficial velocity of the bed is increased, a larger proportion of the gas passes through the bed as bubbles and gives the bed the appearance of a violently bubbling liquid. The local mean bubble size increases rapidly with increasing height above the grid because of coalescence of the bubbles. If the bed vessel is sufficiently narrow and high, the bubbles ultimately fill the entire cross section and pass through the bed as a series of gas slugs. As the gas velocity increases further, more and more solids are carried out of the bed, the original, sharply defined surface of the bed disappears, and the concentration of solids decreases continuously with increasing height. To achieve steady-state operation of such a “turbulent” fluidized bed, solids entrained in the fluidizing gas must be collected and returned to the bed. The simplest way to do this is with cyclone integration into the bed vessel and a standpipe dipping into the bed. A further increase in gas velocity finally leads to the circulating fluidized bed, which is characterized by a much lower average concentration of solids than the previous systems. The high entrainment of solids requires an efficient external solids recycling system with a specially designed pressure seal.
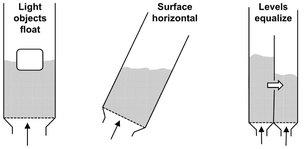
Fig. 4.29: Various characteristics of a fluidized bed. Adapted from [49].
Fig. 4.29 illustrates that the behavior of a fluidized bed in many respects resembles that of a liquid. The bed can be stirred like a liquid. Objects of greater specific gravity sink, whereas those of lower specific gravity float. If the vessel is tilted, the bed surface resumes a horizontal position. If two adjacent fluidized beds with different bed heights are connected to each other, the heights become equal, and the fluidized bed flows out like a liquid through a lateral opening. Particularly advantageous features of the fluidized bed for use as a reactor are uniform temperature and composition due to intensive mixing of particles in the bed, excellent gas-solid contact, good gas-particle heat and mass transfer, and high bed-wall and bed-internals heat-transfer coefficients. These characteristics are a direct consequence of the presence of bubbles in the bed, because the bubbles cause the movement of particles. From this point of view the bubbles are very desirable, but they also cause a reduction in gas contact efficiency with the particles. Therefore, the application of a fluidized bed in a particular process is always a compromise between the advantages of good mixing of solids and the disadvantages of reduced contact efficiency.
4.7.2 Fluidization properties of typical bed solids
In fluidization with gases, solids display characteristic differences in behavior that can also affect the operating characteristics of fluidized bed reactors. As shown in Fig. 4.30, an empirical classification proposed by Geldart can be used to divide solids into four groups (A to D) with respect to fluidization behavior. Solids of group C are very fine-grained cohesive powders (flour, fine dust) that cannot be fluidized without fluidization aids. The adhesion forces between the particles are so strong that the gas will form channels through the bed. Fluidization properties can be improved by the use of mechanical equipment (agitators, vibrators) or flow ability additives. Solids of group A have small particle diameters (± 0.1 mm) or low bulk densities. This class includes catalysts used, for instance, in fluidized-bed catalytic crackers. As the gas velocity increases beyond the minimum fluidization point, the bed of such a solid first expands uniformly until bubble formation sets in. If the gas flow is cut off abruptly, the gas storage capacity of the fluidized suspension causes the bed to collapse rather slowly. Group B solids have moderate particle sizes and densities. Typical representatives are sands with mean particle diameters between 0.05 and 0.5 mm. Bubble formation begins immediately above the minimum fluidization point. Group D includes solids with large particle diameters or high bulk densities. The character of bubble flow is markedly different from that in group B solids. Group D solids are characterized by the formation of “slow” bubbles. On sudden stoppage of the gas flow, the bed also suddenly collapses.
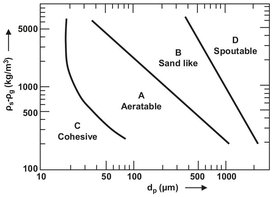
Fig. 4.30: Geldart diagram. Adapted from [47]: for an explanation see the text.
4.7.3 Applications
High temperature homogeneity, even with strongly exothermic reactions and easy solids handling, are the main advantages of fluidized bed reactors over fixed bed reactors. These advantages are achieved at the expense of high solids separation costs for gas purification, low conversion due to intensive mixing, and significant erosion of the internals in a fluidized bed reactor.
The ease of solids handling was the basic reason for the success of long-chain hydrocarbon cracking with zeolite catalysts in the fluidized bed. Because the cracking reaction is endothermic and involves the deposition of carbon on the catalyst surface, the catalyst must be continuously discharged from the reactor and regenerated in an air fluidized regenerator bed. As illustrated in Fig. 4.31, the reaction is carried out in so-called riser cracking reactors, where the oil is fed at the bottom of the riser, vaporized on contact with the hot catalyst, and the mixture of oil vapors and cracking gas transports the catalyst up. In the reactor bed, solids are collected before passing through the stripper to the regenerator.
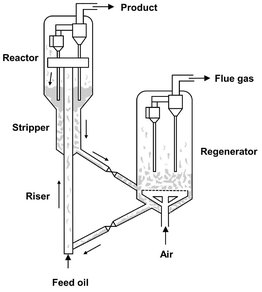
Fig. 4.31: Riser cracking reactor.
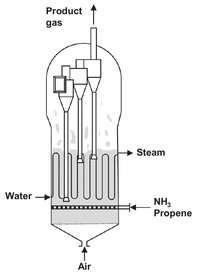
Fig. 4.32: Fluidized bed reactor for the synthesis of acrylonitrile (Sohio process).
The crucial factor in the successful use of the fluidized-bed reactor for the synthesis of acrylonitrile by the ammonoxidation of propene was reliable control of this strongly exothermic reaction:
![]()
The reaction is carried out at a bed temperature of 400-500 °C and gas contact time of 1-15 s. As can be seen in Fig. 4.32, air is fed to the bottom of the reactor and enters the fluidized bed through an air distributor. This gas distribution device ensures uniform fluidization over the entire cross section of the bed and prevents solids from raining through the grid both during operation and after the bed has been shut off. Many different distributor designs are possible, as illustrated by Fig. 4.33. The reactants ammonia and propene are introduced through a separate distributor. This distributor or sparger is designed in such a way that the particles cannot enter the holes, preventing plugging. Catalyst regeneration by carbon burn-off occurs in the space between the air distributor and the feed gas distributor. The heat of reaction is removed by bundles of vertical tubes inside the bed in which high pressure steam is produced at a temperature of around 480 °C. Internal cyclones are used to prevent the entrainment of small catalyst particles from the reactor.
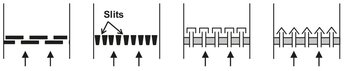
Fig. 4.33: Typical designs of various gas distributors.
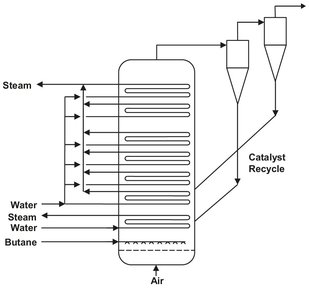
Fig. 4.34: Fluidized bed reactor for the oxidation of butane to maleic anhydride.
Fig. 4.34 shows the fluidized bed reactor used for the synthesis of maleic anhydride from n-butane by partial oxidation over a V2O5 catalyst:
![]()
Compressed air and butane are introduced separately into the bottom of the reactor. Heat from the exothermic reaction is removed from the fluidized bed through steam coils in direct contact with the bed of fluidized solids. Due to the high mixing in the bed, a uniform temperature profile is obtained, which is an important precondition for high reaction selectivity. The solids are removed from the product gas using a combination of cyclones and filters. One particular problem of this process is the mechanical stress on the catalyst, and its abrasion and erosion at the heat-dissipating surfaces.
The gas-phase polymerization of ethylene in the fluidized bed shown in Fig. 4.35 was developed by Union Carbide. The reaction gas fluidizes the bed at 75—100 °C and a pressure of 20 bar. Extremely fine-grained catalyst is metered into the bed. Polymerization occurs on the catalyst surface and yields a granular product with diameters ranging from 0.25 to 1 mm. Temperature control is achieved by keeping the conversion per pass low, around 2 %, and recycling the gas over an external heat exchanger. The catalysts have such a high activity that removal from the final product is not necessary.
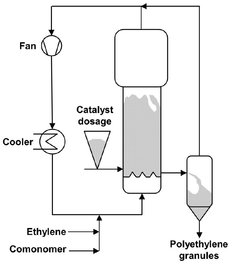
Fig. 4.35: Gas-phase polymerization of ethylene.
Other important fluidized bed reactor processes are the low-pressure synthesis of melamine from urea, oxidation of naphthalene to phthalic anhydride, the Exxon Flexi-Coking process, coal gasification, and many others.