Process Technology: An Introduction - Haan A.B. 2015
5 Biochemical reaction technology
5.1 Characteristics of biochemical processes
Biochemical reactions involve the transformation and production of biological and chemical substances. Typical of these reactions is their use of enzymes as biocatalysts. In addition to their natural substrates, many of these enzymes can utilize other structurally related compounds as substrates and therefore catalyze unnatural reactions upon addition of foreign substrates to the reaction medium. Thus enzymatic conversion is a specific category of chemical synthesis. The enzymes can be present as cell constituents of living microorganisms, or they can be isolated in dissolved form or bound to inert supports. At present, fermenting microorganisms are still the dominant and least costly form of industrial biological catalyst production. Their great advantage is their versatility, as illustrated by the wide range of the processes in current use. However, fermentation requires complex and often expensive nutrients that are mainly used as an energy source. Continuous operation may also be difficult with fermentation because of strain mutation.
Tab. 5.1 presents a comparison between chemical processes and bioprocesses such as simple fermentation. The analysis is based on the characteristics of the catalysts. When compared with chemical catalysts, enzyme catalysts are both highly active and highly selective. Additional advantages include the variety of reactions catalyzed, the high degrees of conversion, and the mild conditions employed, which is especially important when labile reactants are used. Today, the use of enzymes in the whole cell as the catalyst is preferred.
Tab. 5.1: Comparison of bioprocessing and chemical processing.
Criterion of comparison |
Chemical process |
Bioprocess |
|
Catalyst |
More or less active and selective Expensive regeneration |
Enzymes highly active and selective |
Regeneration easy due to microbial growth |
||
Reaction conditions |
Mostly high temperatures, sometimes high pressures |
Mainly 25 °C and 1 bar pressure |
Raw materials |
Pure in most cases |
Impure, inactive, and diluted |
|
Process |
Often multistage processing with recovery of intermediates |
One-stage possible without intermediate product recovery |
Fast reaction rates at high concentration level and high yields |
Mainly slow reaction rates Risk of infections and mutations |
5.1.1 Fermentation
Fermentation is a long-established process which has expanded over the years to become the basis of biotechnology and biochemical engineering. Many new applications have been discovered in recent decades, such as the mass production of secondary metabolites (antibiotics), biotransformations of organic substances such as steroids, mass cultivation of microorganisms for enzyme production, and the marked impact of genetic engineering. Biochemical conversions with the aid of a microorganism differ from the purely chemical process in several aspects:
· — complexity of the reactant mixture;
· — increase in the mass of microorganisms simultaneously with the accomplishment of the biochemical transformation;
· — ability of microorganisms to synthesize their own catalysts (enzymes);
· — mild conditions of temperature and pH involved, and its greater sensitivity to these conditions;
· — restriction to the aqueous phase.
5.1.1.1 Fermentation products
Microorganisms are exploited industrially to produce additional microorganisms and to achieve biochemical conversion of water-soluble organic compounds. The microbial cells are used as animal foodstuffs, for seeding batch processes, and as a source of enzymes for both research and commercial applications. The biochemical products on the other hand may be beverages, medicinal compounds such as antibiotics and steroids, and industrial chemicals, e.g. solvents or organic acids.
Microbial fermentations are an important source of biological products used in the pharmaceutical, food, and chemical industries. During the last decade there has been a large increase in the range of commercial products, especially secondary metabolites and recombinant proteins. Among these products, cells, primary metabolites, secondary metabolites, and enzymes can be distinguished as the main products. Yeast fermentation for brewing and baking represents the most traditional form of fermentation technology in which the cell is obtained as the main product. Primary metabolites such as citric acid, lactic acid, ethanol, and glutamic acid are produced in very large-scale operations. Because of their relatively simple metabolics, these processes are relatively well characterized. Stirred tank fermenters of around 250—750 m3 in scale are generally used. If it is desired to go to greater volumes, then it is simpler to switch to an air-lift type design because of heat transfer limitations.
Since the widespread use of antibiotics began, this area has represented the most commercially significant fermentation activity for secondary metabolites. Products such as penicillin, tetracycline, erythromycin, cephalosporins, cephamycins, and clavulanic acid are made at a very large scale in fermenters varying between 50 to 200 m3 in volume. In most cases these products serve a vital role in modern medicine. Secondary metabolite fermentations are very complex and have yet to yield many of their secrets.
Historically, industrial enzymes made by fermentation were mainly restricted to those produced extracellularly, such as amylases and proteases. With the development of mechanical techniques for protein release from microorganisms on a larger scale, intracellular enzymes have found wider application in the food, pharmaceutical, and chemical industries. Some important examples are glucose isomerase for high-fructose syrup production and penicillin acylase for removal of the side chain of penicillins to allow subsequent manufacture of semisynthetic antibiotics. Fermenter sizes for enzyme production generally range from 30 to 220 m3.
5.1.1.2 Microorganisms
The living cells used in bioreactors can be bacteria, yeasts, moulds, and plant or animal cells (Fig. 5.1). This broad classification is made on a basis of cell size and morphology when examined with an optical microscope. Bacteria are single-cell microorganisms with their smallest dimension in the range of 0.5 to 2 µm, and they reproduce asexually by binary fision. Yeasts, on the other hand, while also unicellular, have a size range from 5 to 10 µm and reproduce either by asexual budding or by sexual processes. Moulds may also reproduce by sexual or asexual means, but have a multicellular structure and may be 5 µm or considerably larger in size. Plants and animal cells are fragile and relatively large. They grow slowly, making great demands on nutrient supply. As a result of their large size (50—100 µm), complex structure, and mechanical sensitivity, nutrient media have to satisfy high demands, and the bioreactor must fulfil special requirements.
Bacteria have an optimum pH range for growth between 6.5 and 7.5 and display a wide variety of patterns of response to free oxygen. Some have absolute oxygen requirement, while others can grow only in its complete absence. Intermediate species exist that develop with or without oxygen, though not necessarily at the same rates. Moulds however, grow most rapidly under aerobic conditions, but generally more slowly than bacteria. They can therefore be overgrown by bacterial contaminants. An abundant supply of oxygen is required for the growth of yeasts. The acid tolerance of yeast fermentations ranges from pH 2.2 to pH 8.
As illustrated by Fig. 5.2, microbial metabolism maybe thought of as a series of interconnecting reaction loops or metabolic pathways arranged spatially throughout the cell. The basic unit in a metabolic pathway is a reaction catalyzed by an enzyme. The overall reaction path is controlled by the microorganism itself, largely by adjustment of the rate of enzyme synthesis, or alternatively by inhibition of the enzymes by the product itself. The industrial objective is to use a part of the overall metabolism for a particular biochemical conversion. An example is glutamic acid that can be obtained as a primary metabolite from the Krebs cycle shown in Fig. 5.2. This is achieved by the supply of a primary organic raw material, called substrate, in addition to those metabolites required by the micro-organism for survival. If possible, an attempt is made to enhance the amounts and activity of the enzymes involved in the conversion, while those enzymes acting on the desired product are inhibited or repressed. These objectives are attained by the addition of appropriate chemical constituents to the reaction mixture or nutrient medium. In contrast to isolated enzymes or chemical catalysts, micro-organisms adapt the structure and activity of their enzymes to the process conditions, whereby selectivity and productivity can change. Mutations of the micro-organisms can occur under suboptimal biological conditions. Microorganisms are frequently sensitive to strong shear stress and to thermal and chemical influences.
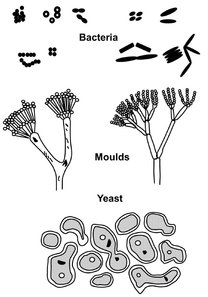
Fig. 5.1: Morphological characteristics of microorganisms.
The type and metabolic state of the microorganisms used are decisive for the initial choice of reactor. Most modern biotechnological methods are based on aerobic processes. In the cultivation of aerobic microorganisms, the culture medium must always contain sufficient dissolved oxygen to allow the biochemical reactions of the microorganisms to occur. Oxygen is only sparingly soluble in the fermentation medium, making the supplying of microorganisms with this nutrient technically very complex. In aerobic processes, an effective and adequate oxygen supply must be provided in the medium by dispersing and subsequent mixing in all zones of the reactor.
5.1.1.3 Requirements of fermenters
In most bioprocesses, product processing is the cost-determining step, with the result that the aim of the reactor design is to obtain the maximum product concentration. To achieve this goal, bioreactors with capacities up to thousands of cubic meters are used. Fermenters are classified into those employing anaerobic or aerobic conversions with free microorganisms. Many different reactor configurations are employed, depending on the microbial structure. The basic reactor arrangement for the accomplishment of fermentations is the deep tank fermenter shown in Fig. 5.3. The basis of this reactor is the fact that microorganisms in their normal state contain a considerable amount of water and have a density that differs only slightly from water. Very little hydrodynamic drag is required to maintain them in suspension. The logical arrangement then becomes an essentially completely mixed reactor in which the motion is either by mechanical stirring or by air bubbling through the medium. This air bubbling provides the free oxygen demanded by aerobic processes.
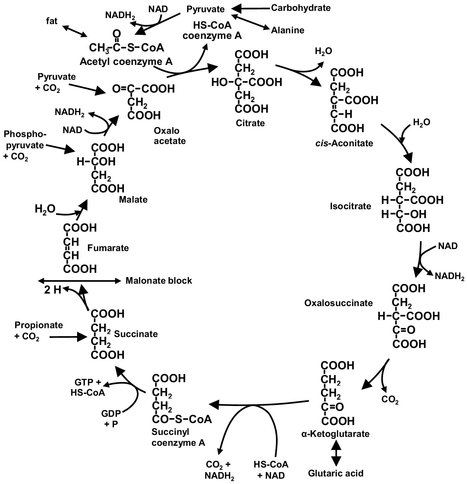
Fig. 5.2: Schematic of the Krebs cycle.
The prerequisite for the use of living microorganisms in reactors is the provision of favorable living conditions, to ensure that living microorganisms can exhibit their activity under defined conditions. This results in a series of special features in the reaction engineering of biocatalytic processes. The reaction rate, cell growth, and process stability depend strongly on the environmental conditions in the bioreactor. Concentrations of substrates and products in the reaction mixture are frequently low, and both of them may inhibit the process. Cell growth, the structure of intracellular enzymes, and product formation depend on the nutritional needs of the cell and on the maintenance of optimum biological conditions within narrow limits. Certain substances (inhibitors, effectors, precursors, metabolic products) influence the rate and the mechanism of the reactions and intracellular regulation. Microorganisms can metabolize unconventional or even contaminated raw materials (cellulose, molasses, mineral oil, starch, ores, waste-water, exhaust air, biogenic waste). General fermenter requirements involve monoseptic conditions, containment, optimal mixing with low uniform shear, adequate oxygen transfer, feeding of substrate with prevention of under— or overdosage, suspension of solids, gentle heat transfer, and compliance with design requirements.
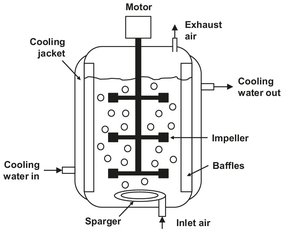
Fig. 5.3: Schematic of the deep tank fermenter.
5.1.2 Enzymatic conversions
5.1.2.1 Industrial applications
Enzymes accelerate hundreds of reactions taking place simultaneously in the cell and its immediate surroundings. Enzyme processes enable natural raw materials to be upgraded and turned into finished products. They offer alternative ways of making products which previously could be made only by conventional chemical processes. The detergent industry is the largest user of industrial enzymes. The starch industry, the first significant user of enzymes, developed special high fructose syrups that could not be made by means of conventional chemical hydrolysis. These were the first products made entirely by enzymatic processes. Foodstuffs and components of animal feed can be produced by enzymatic processes which require less energy, less equipment, or fewer chemicals compared to traditional techniques.
During the last decade, the role of biocatalysis in organic synthesis, biochemical and biomedical processes has increased dramatically. An example of a very large-scale application is the isomerization of glucose to fructose with glucose isomerase. Other important processes where enzymes are used on scale over 1000 t/yr include the synthesis of aspartame and the enzymatic hydrolysis of penicillin G. In the aspartame process the enzyme thermolysine is used for the enantioselective condensation of the two amino acid constituents (Z-L-Asp and L-Phe-Ome). Penicillin G acylase is used for the selective hydrolysis of penicillin G and penicillin V to obtain 6-aminopenicillanic acid (6-APA), which is an important building block for semisynthetic antibiotics. The need for enantiomerically pure compounds in the pharmaceutical, food, and cropprotection industries, owing to consumer and regulatory demands, will continue to fuel the interest in biocatalysts and associated processes.
5.1.2.2 Distinctive features of enzymes
Enzymes are very powerful biological catalysts, catalyzing all of the reactions that constitute cellular metabolism. They consist of L-amino acids linked together by covalent bonds in a defined sequence that is coiled in a complex fashion. The active site consists of only a few amino acids that have a direct role in binding the substrate and catalyzing the reaction characteristic of each particular form of enzyme molecule.
Enzymes are very efficient, catalyzing reactions often 108—1011 times more rapidly than the corresponding chemical catalysts. Most of the reactions proceed in water at much less extreme conditions, such as temperature, pH, and pressure. The range of reactions that can be catalyzed by enzymes is extremely broad. Most enzymes are very specific in terms of the type of reactions catalyzed and the structures of the substrate and product formed, such that often only a single chemical present in a mixture with very similar chemicals is transformed into a single product. This can result in higher product yields and fewer potentially polluting side-products. This specificity is due to the ability of the enzyme to bind the substrate and organize reactive groups so that a specific reaction transition is particularly favored. Several main types of enzymatic catalysts can be recognized, including whole cells, organelles, and enzymes, used in both free and immobilized forms. Any combination of these systems may also be possible. Although enzymes are sufficiently large to be regarded as heterogeneous catalysts, they are usually classed as being homogeneous catalysts due to their solubility. Obviously, immobilized enzymes in which the particle size of the catalyst is at least one order of magnitude larger than the enzyme are genuine heterogeneous catalysts.
5.1.2.3 Enzymatic catalysis
Enzymes are classified into six different groups depending on the type of reaction they catalyze:
· — oxidoreductases catalyze oxidation-reduction reactions involving oxygenation, such as C—H → C—OH, or overall removal or addition of hydrogen atom equivalents, for example CH(OH) → C=O;
· — transferases mediate the transfer of various groups, such as aldehyde, ketone, acyl, sugar, phosphoryl and so on from one molecule to another;
· — hydrolases can act on a very broad array of hydrolysable groups. It includes esters, amides, peptides and other C—N-containing functions, anhydrides, glycosides, and several others;
· — lyases catalyze the addition of groups to double bonds such as C=C, C=O, and C=N, or the reverse;
· — isomerases transfer groups within molecules to yield isomeric form such as racemization;
· — ligases are often termed synthesases; they mediate the formation of C-O, C—S, and C—N bonds.
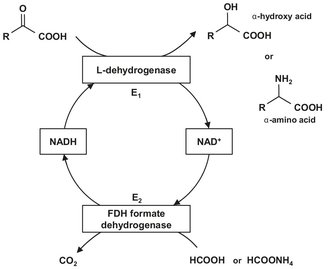
Fig. 5.4: Example of cofactor regeneration of NAD+/NADH.
Not all enzymes are capable of acting alone. Many require the presence of nonprotein cofactors to enable catalysis to be carried out. Such cofactors, which are in reality cosubstrates because they undergo chemical transformation during the reaction, include simple metal ions or organic molecules that may in some cases be covalently bound to the enzyme. They must be continuously reconverted back to their original form in order for catalysis to continue. This process, illustrated by Fig. 5.4, is usually referred to as regeneration. Regeneration can take place spontaneously in aerobic aqueous conditions by hydrolysis or oxidation reactions. However, in most cases, including ATP, coenzyme A, folic acid, NAD+, and NADP+, regeneration can only be achieved by directly coupling with the oxidation of high energy substrate molecules, either by cytochromes or by enzymes. In the whole cells regeneration is an aspect of the normal integrated metabolism of the cell, via substrate level and cytochrome linked exergonic reactions. However, when isolated enzymes or disrupted cells are used, regeneration may be a considerable problem.
In industry enzymes or cells are often used in an immobilized form to enable reuse or continuous use of the biocatalyst and easy product recovery. Continuous use is especially important for maintaining a constant environment for the immobilized biocatalyst and thereby improving enzyme stability. Numerous immobilization methods are used. They can be divided into the following three groups:
· — entrapment or encapsulation in a porous polymer network;
· — covalent, ionic, or physical attachment to an appropriate water-insoluble solid support;
· — aggregation of cells by physical or chemical cross-linking with glutaraldehyde or other agents.
Although immobilization tends to increase the stability of the enzyme or cell, usually some enzyme activity is lost due to denaturation during immobilization. With immobilized cells, flow rates through the reactor and/or the concentration of the enzyme can be increased. As a result a much smaller reactor can be used to achieve the same productivity, reducing the capital and operating costs of enzyme catalyzed processes. The choice between the use of immobilized enzymes or immobilized cells is similar to the choice between the use of purified or crude soluble enzymes as a biocatalyst. Clearly the immobilized cell or crude enzyme is cheaper, and larger quantities are available, but they are less specific catalysts than immobilized enzymes or the purified soluble enzymes.