Process Technology: An Introduction - Haan A.B. 2015
5 Biochemical reaction technology
5.2 Biochemical reaction engineering
5.2.1 Principles
As with chemical reactors, the calculation of microbiological and biochemical processes is based on the balance equations of the bioreactor. Depending on their state of mixing, bioreactors are denoted as ideally mixed stirred tank reactors or ideal plug-flow reactors. To solve the balances, the stoichiometry, kinetics, and energetics of the biochemical process must be known and understood. In this modelling the kinetics of microbial growth and product formation or material transformation by the enzymes or microorganisms are of central importance.
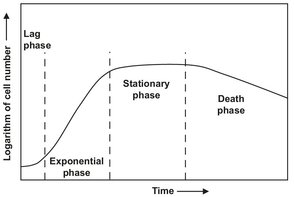
Fig. 5.5: Growth curve of a batch bacterial culture.
5.2.2 Kinetics of biochemical reactions
5.2.2.1 Microbiological processes
In many respects the bacterial growth process is similar to a chemical reaction in which the components of the medium produce cells in addition to excreted products. Fig. 5.5 shows that after inoculation of a batch reactor the bacteria start to grow —slowly in the beginning and exponentially at their maximum rate during the major part of the growth period. In this characteristic exponential phase the growth rate is autocatalyzed by the bacterial population itself. The rate of growth decreases and then ceases, and finally the cells start to die.
Generally the bacterial growth rate remains virtually constant until the medium is almost exhausted of the limiting nutrient. This seeming paradox is explained by the action of enzymes (permeases) which are capable of maintaining constant intracellular concentrations of substrates and nutrients over a wide range of external concentrations. Nevertheless, usually at extremely low concentrations of external nutrients the permease enzymes are no longer able to maintain the intracellular concentrations, and the growth rate falls. The curves relating growth rate to substrate concentration are typically hyperbolic in form (Fig. 5.6) and can be described by the Monod equation
![]()
(5.1)
where µ is the specific growth rate (s-1), S the substrate concentration (mol/m3), µmax the growth rate at infinite concentration of the substrate, and Ks (mol/m3) the Monod uptake constant for the substrate.
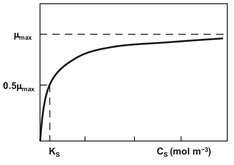
Fig. 5.6: Effect of substrate concentration on the specific growth rate In case of Monod kinetics.
5.2.2.2 Enzyme catalyzed reactions
Enzyme catalysts are subject to the same kinetic and thermodynamic constraints as are chemical catalysts. They alter the rate at which a reaction proceeds, but not the final position of equilibrium between the substrate (S) and product (P). Catalysis is effected by enabling an alternative reaction mechanism and thus an alternative transition state with a lower free energy, by lowering the free energy of the conventional transition state intermediate, or by providing an environment that decreases the free energy of the product.
In the course of an enzyme reaction, generally three phases are recognized. At the start, most of the free enzyme combines with substrate in a dynamic equilibrium that persist as long as fresh substrate molecules are available. In the second phase, all the reactants, including the enzyme molecules, are in a dynamic equilibrium with the maximum activity being expressed. In the final stage of the reaction, the substrate concentration is strongly reduced, and thus the rate of the enzyme catalyzed reaction falls asymptotically. This phase of reaction is especially important in many industrial reactions where complete conversion is desired. It can often occupy the majority of the reaction time, especially when product inhibition takes place, which is especially likely when high substrate concentrations are employed. The kinetics of enzyme reactions can be derived on basis of the following general reaction scheme for conversion of a single substrate (S) to a single product (P):
![]()
(5.2)
When the concentration of substrate is much greater than the concentration of enzyme [E] the rate of reaction depends only on the concentration of enzyme present. Thus the velocity of the reaction remains essentially constant until nearly all the substrate has been consumed. This kinetic behavior is reasonable well described by the well-known Michaelis—Menten relationship (Fig. 5.7):
![]()
(5.3)
where r is the reaction rate, E0 the enzyme concentration, and KM = (k-1 + k2)/k1 the Michaelis constant representing the substrate concentration which gives half the maximum rate of reaction.
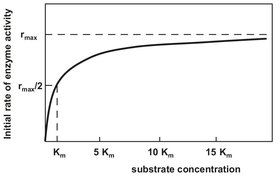
Fig. 5.7: Plot of the Michaelis—Menten enzymatic reaction rate.
5.2.2.3 Environmental effects
In general the pH of the reaction medium has a pronounced effect on both enzyme and cell kinetics. The activity as a function of pH generally displays a bell-shaped profile, as shown in Fig. 5.8. This definite optimum pH for enzyme activity is usually observed because enzymes contain many ionizable groups. As a result, pH changes alter the conformation of the enzyme and thereby the binding of the substrate and the catalytic activity. The overall effects may be observed by a change in the maximum reaction rate (rs,max), a change in the affinity of the enzyme for the substrate (Km), or an alteration in the stability of the enzyme. In a similar fashion ionizable groups in the substrate can be affected by pH and alter the enzyme-substrate complex formation process.
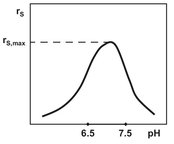
Fig. 5.8: Effect of pH on enzymatic activity.
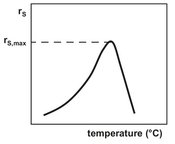
Fig. 5.9: Enzymatic activity as a function of temperature.
The effects of temperature on enzyme and cell kinetics generally exhibit a rather simple behavior. The overall rate passes through a maximum with a sharp decrease at higher temperatures (Fig. 5.9). This behavior is the result of a combined effect. Raising the temperature affects the enzyme-catalyzed reaction as well as the thermal inactivation of the enzymes. Although the underlying mechanisms are very complex, the rate of enzyme catalysis as well as the rate of enzyme denaturation can be described satisfactorily with the Arrhenius equation
![]()
(5.4)
Activation energies for enzyme catalyzed reactions are normally in the range of 4—20 kcal/mol such that the rate of reaction increases only slowly with an increase in temperature. Enzyme stability is much more strongly influenced by temperature because activation energies of an enzyme denaturation range of 40—130 kcal/mol. The optimum temperature for a reaction to take place is a compromise between these dual effects of increased temperature.
5.2.2.4 Inhibition
Four common types of inhibition are recognized. Irreversible inhibition occurs when the inhibitor molecule combines irreversibly with the enzyme, chemically modifying its structure and destroying its catalytic activity. As illustrated in Fig. 5.10, three types of reversible inhibition can occur:
· — competitive, which can be reduced by increasing the substrate concentration:
![]()
(5.5)
· — noncompetitive, which cannot be reduced:
![]()
(5.6)
· — excess substrate inhibition, caused by the formation of nonproductive complexes that can be decreased by using a lower substrate concentration:
![]()
(5.7)
Competitive inhibitors compete with the normal substrate molecules to occupy the active site of the enzyme forming a reversible enzyme inhibitor complex which is characterized by the Ki value, the dissociation constant of this complex. With noncompetitive inhibition, the inhibitor also forms a reversible complex with the enzyme but at a site other than the active site that is not reduced by increasing the substrate concentration. Inhibition by substrate is less common, but it occurs at high substrate concentration, even though inhibition is not readily apparent, and Michaelis—Menten kinetics are obeyed at low substrate concentrations. Inhibitors are often present in low concentrations in many substrates, and inhibition may only become serious when continuous high throughputs of substrate are used.

Fig. 5.10: Schematic representation of inhibition.
5.2.3 Basic reactor operations
Like chemical reactors, bioreactors are operated in batch and continuous mode. In addition several semicontinuous modes are frequently employed in biotechnological production:
· — fed-batch operation with varying feed rates and volume-time patterns in the reactor;
· — extended fed-batch operation with continuous feeding, the substrate concentration remaining constant;
· — repeated (fed)-batch, where after the batch reaction a small amount of the fermentation broth is left in the reactor as inoculum for the next batch or fed-batch process.
Batch biotechnological processes are characterized by inoculation of the sterile culture medium with microorganisms, cultivated for a specific reaction period. During the reaction, cell, substrate, and product concentrations alter. Good mixing ensures that there are no significant local differences in the composition and temperature of the reaction mixture. As the oxygen in the culture medium is slightly soluble, a continuous oxygen supply is needed for aerobic cultivation while removing the CO2 formed in the same way. This is generally done by aeration of the medium. Acid or alkali is added periodically to the system to control the pH value. Antifoaming agents are also added for chemical foam suppression. The main advantage of batch bioreactors is the low risk of infection and cell mutation due to relatively short cultivation periods. Other advantages of batch operation are low investment costs, greater flexibility, and higher conversion levels. The disadvantages of batch operation are the nonproductive idle time, higher operating costs, and the greater risk of contact with pathogenic organisms or toxic products. Hence, batch reactors are used in biotechnology only when small amounts of product are involved, when there is a high risk of infection or microorganism mutation, or when downstream processing is discontinuous.
In continuous operation the culture medium is fed continuously into the bioreactor, and the reaction mixture is also drawn continuously from the reactor. The medium added may be sterile, or it may contain the microorganisms or enzyme used. All reaction variables and control parameters remain constant in time. As a result, a steady state is established in the reactor, characterized by constant productivity and output. The main advantages of continuous bioreactors are lower reactor volume, constant product quality, and less possible danger from pathogenic microorganisms or toxic materials. However, continuous operation has a high risk of infection or microorganism mutation due to long cultivation periods. Therefore, continuous operation is preferred for processes with high production rates, for gas, liquid, or soluble solid substrates, and when microorganisms with a high mutation stability are involved.
Semicontinuous operation can be regarded as a combination of batch and continuous operations. Many variations of this type of process are practiced. The most popular involves starting the reactor as a batch process until the growth-limiting substrate is consumed, which is subsequently fed to the reactor in a specified manner (fed-batch) or kept constant in concentration by extended culture. Moreover, programmed substrate addition is frequently practiced for secondary metabolite production in which cell growth and product formation occur in separate phases. Semicontinuous processes attempt to combine the advantages of continuous and batch operation. They have high flexibility and a high yield, but still suffer from the nonproductive idle time and risk of contact with pathogenic microorganisms or toxic products. It is clear that semicontinuous processes are often used when continuous methods are not possible (due to slight mutation or infection of microorganisms) and batch production would result in low productivity figures. Like batch reactors, semicontinuous reactors are nonstationary.