Process Technology: An Introduction - Haan A.B. 2015
5 Biochemical reaction technology
5.3 Industrial bioreactors
5.3.1 Classification
Bioreactors are vessels in which raw materials are biologically converted into specific products, using microorganisms, plant, animal, or human cells, or individual enzymes. A bioreactor supports this process by providing suitable conditions such as optimum temperature, pH, sufficient substrate, nutritional salts, vitamins, and oxygen. The design of bioreactors depends on the biomass concentration, sterility requirements, mixing, suspension, aeration, heat transport, and the generation of optimum shear conditions. For microbial as well as enzymatic conversion reactors good heat removal systems have to be employed, because the temperature of the fermentation process should be maintained at an optimum, constant value. Bioreactions with a sensitive pH optimum should be conducted in reactors with a high mixing intensity and a distributed feed system for agents that prevent local high concentrations of acids or bases. Unstable or toxic intermediates or products can be withdrawn from the reaction mixture by an integrated separation step. Enzyme reactors differ from chemical reactors because they function at low temperatures and pressures, and comparatively little energy is consumed or generated during reaction. Enzyme reactors differ from fermentations in that they do not behave in an autocatalytic fashion.
Biochemical processes are either aerobic or anaerobic, and each type demands a different set of conditions. Aerobic reactors can be subdivided into submerged and surface reactors. The difference is that in surface reactors microorganisms are supplied with oxygen through the surface of a medium, which generally forms a thin film on the solid carrier. In submerged reactors the necessary oxygen and substrate are supplied to microorganisms by air dispersion and subsequent intensive bulk mixing of the medium and dissolved oxygen. Submerged reactors are more suitable for mass production and have therefore become more popular over the last few decades.
A frequently used and practical classification of submerged bioreactors is based on the type of energy supply. There are a number of ways in which turbulence can be produced by energy dissipation. In some reactors, energy is generated by mechanically moving agitators. The stirred-tank reactor, the best known in this category, has been regarded as the classic example of a biotechnological reactor. However, it is also true that over the same period cases have appeared for which the standard stirred tank reactor is not suitable. Thus it might be uneconomic to operate or unsuitable for biological reasons. Large numbers of new types of reactors have been developed and modifications to existing models made in attempts to provide alternatives that offer technical or economic advantages for special production processes or aerobic effluent treatment. These new variations offer alternatives to mechanical energy input. Some examples are reactors with an external liquid pump and reactors with no mechanical parts, where gas expansion through a sparger provides the required energy for mixing.
5.3.2 Bioreactors with mechanical mixing
The stirred-tank reactor is still the most important type of reactor in biochemical technology. Mechanically stirred bioreactors have the following functions: homogenization, suspension of solids, dispersion of liquid-liquid mixtures, gassing/aeration of the liquid, heat exchange, and influencing microorganisms through shear. Homogenization is the most important aim of mechanical agitation in nonaerated submerged reactors. As the density of the microorganisms is almost the same as that of water, forming a suspension is not very difficult. On the other hand, gas-phase dispersion (usually air) in cultivation media is a very significant aspect of aerobic submerged cultures. As the required reactor size increases, the more difficult it is to obtain uniform aeration and adequate homogenization and heat removal. In small aerobic reactors (< 1 m3) the main function of a mechanical agitator is gas dispersion. Above this level (> 10 m3), homogenization, aeration, and heat removal are of equal importance. Heat is generated by biological and agitation processes, and thus the intensification of heat removal by mechanical agitators is a vital aspect.
A simple stirred-tank fermenter is presented in Fig 5.11. As a rule, the tank is provided with four baffles. Typical stirrers for low-viscosity media are the propeller and pitched-blade turbine for axial motion and the disk and impeller stirrer for radial motion. In moderately to highly viscous media, other stirrer types are used. Multiple stirring devices are have advantages for mixing highly viscous media such as mycelia fermentations used in producing antibiotics. Heat transfer in stirred-tank reactors is achieved by use of double jacket, helical, or meander coils, or by external heat exchangers.
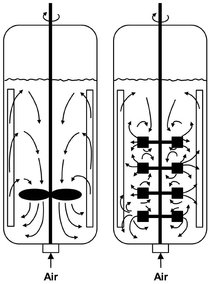
Fig. 5.11: Schematic of single— and multiplestirrer reactors.
5.3.3 Bioreactors with pneumatic mixing
In this type of reactor, compressed air is expanded and dispersed through a gas distributor. As the density of the gas is considerably lower than that of the medium, gas bubbles rise up and liquid is entrained with them. The power supplied by the compressed gas disperses the gas and mixes the medium at the same time. Bioreactors with pneumatic mixing have the advantage of simple design, good heat transfer, small base area, low-maintenance operation, and very low shear forces. They can be classified into bubble-column reactors and airlift reactors.
5.3.3.1 Bubble-column reactors
These reactors (Fig. 5.12) are comprised of vertical columns fitted with a static gas distributor (air sparger) at the base of the column and different design variations to improve gas dispersion and residence time. To prevent heterogeneous flow patterns in the lower compartment, the sparger nozzles have to be distributed over the cross section of the bottom. Although their construction is very simple, the flow patterns inside them are highly complex, and detailed design specifications are required for optimum operation. This is largely due to the fact that the properties of the media change greatly during the reaction and cause process engineering problems due to foam formation, biomass flotation, and bubble coalescence.
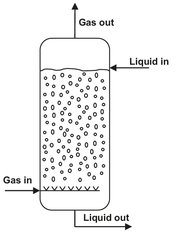
Fig. 5.12: Bubble column.
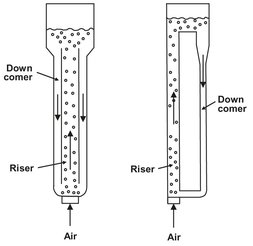
Fig. 5.13: Air lift reactor with internal and external loop.
5.3.3.2 Airlift reactors
In airlift reactors (Fig. 5.13), the circulation of the fermentation liquid is provided by the density difference between the aerated liquid in the tower and the nonaerated liquid in the loop. They may have internal or external circulation. Internal circulation can be produced by two coaxial draft tubes or flow inversion by gassing the outer cylinder space. Perforated plates, static mixers, or packing may be installed for multiple dispersion of the gas bubbles. Airlift reactors with external circulation exhibit favorable flow behavior, because heat exchangers or additional mixing elements can be installed in the downcomer. Airlift reactors are especially suitable for shear-sensitive, flocculating, and foaming fermentation systems. Volumes can be up to thousands of cubic meters.
The air lift consists of two pipes, interconnected at top and bottom. In one of the pipes (riser), air is sparged at the bottom. The air rises and escapes at the top. Therefore, under most circumstances there is no air present in the other pipe (downcomer). The density difference between riser and downcomer causes an intensive liquid circulation. The aerated liquid with a lower density level reaches a higher surface level than the nonaerated liquid, which has a lower gas content and greater density. Hence the surface of the aerated phase in the riser should be above that of the nonaerated liquid phase in the downcomer. Liquid passing through the closed loop moves from the higher to the lower lever, thus establishing a circulation pattern.
5.3.4 Bioreactors for immobilized enzymes and cells
Three techniques have been developed for increasing the biocatalyst concentration in the reactor and thus retention for continuous process operation. Separation of the biocatalyst at the reactor outlet, retention of the biocatalyst by micro— or ultrafiltration and immobilization. The reactors are considerably smaller than those for free enzymes and cells due to the concentration of the biocatalyst. Examples of bioreactors that employ immobilized enzymes and cells are shown in Fig. 5.14. They include fixed-bed reactors (glucose isomerization, 1-amino acid production), staged fixed-bed reactors, and stirred slurry reactors.
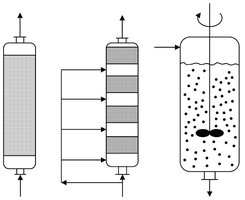
Fig. 5.14: Schematic of fixed-bed, multiple fixed-bed and slurry bioreactors employing immobilized enzymes and cells.
Nomenclature
E |
enzyme concentration |
[mol m-3] |
EA |
activation energy |
D mol-1] |
El |
enzyme-inhibitor complex concentration |
[mol m-3] |
ES |
enzyme-substrate complex concentration |
[mol m-3] |
l |
inhibitor concentration |
[mol m-3] |
k |
reaction rate constant |
[s-1] |
KM |
Michaelis constant |
[mol m-3] |
KS |
Monod substrate uptake constant |
[mol m-3] |
P |
product concentration |
[mol m-3] |
r |
reaction rate |
[mol m-3 s-1] |
R |
gas constant (8.3144) |
[J mol-1 K-1] |
S |
substrate concentration |
[mol m-3] |
T |
temperature |
[K] |
µ |
specific growth rate |
[s-1] |
Indices
A |
species A |
max |
at infinite substrate concentration |
0 |
initial |
S |
substrate |
* |
nonproductive |