Process Technology: An Introduction - Haan A.B. 2015
6 Evaporative separations
6.1 Evaporative separation
6.1.1 Introduction
A large part of the separations of individual substances in a homogeneous liquid mixture or complete fractionation of such mixtures into their individual pure components is achieved through evaporative separations. Evaporative separations are based on the difference in composition between a liquid mixture and the vapor formed from it. This composition difference arises from differences in effective vapor pressures, or volatilities, of the components in the liquid mixture. The required vapor phase is created by partial evaporation of the liquid feed through adding heat, followed by total condensation of the vapor. Due to the difference in volatility of the components the feed mixture is separated into two or more products whose compositions differ from that of the feed. The resulting condensate is enriched in the more volatile components, in accordance with the vapor-liquid equilibrium for the system at hand. When a difference in volatility does not exist, separation by simple evaporation is not possible.
The basis for planning evaporative separations is knowledge of the vapor-liquid equilibrium. Technically, evaporative separations are the most mature separation operations. Design and operating procedures are well established. Only when vaporliquid equilibrium or other data are uncertain is a laboratory and/or pilot-plant study necessary prior to the design of a commercial unit. The most elementary form is simple distillation, in which the liquid mixture is brought to boiling, partially evaporated, and the vapor which has formed separated and condensed to form a product. This technique is commonly used in the laboratory for the recovery and/or purification of products after synthesis in an experimental setup, as illustrated by Fig. 6.1.
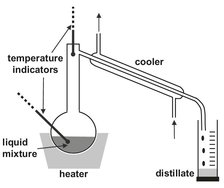
Fig. 6.1: Laboratory distillation setup.
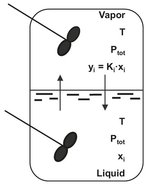
Fig. 6.2: Vapor-liquid equilibrium schematic.
6.1.2 Vapor-liquid equilibria
It is clear that equilibrium distributions of mixture components in the vapor and liquid phase must be different if separation is to be made by evaporation. At thermodynamic equilibrium the compositions are called vapor-liquid equilibria (VLE), as depicted in Fig. 6.2. For binary mixtures the effect of distribution of mixture components between the vapor and liquid phases on the thermodynamic properties is illustrated in Fig. 6.3. Fig. 6.3a shows a representative boiling point diagram with equilibrium compositions as functions of temperature at a constant pressure. Commonly, the more volatile (low boiling) components are used to plot the liquid and vapor compositions of the mixture. The lower line is the liquid bubble point line, the locus of points at which on heating a liquid forms the first bubble of vapor. The upper line is the vapor dew point line, representing points at which a vapor on cooling forms the first drop of condensed liquid. The region between the bubble and dew point lines is the two-phase region where vapor and liquid coexist in equilibrium. At the equilibrium temperature Te the liquid with composition xe is in equilibrium with the vapor composition ye. Fig. 6.3b displays a typical isobaric y-x diagram that is obtained by plotting the vapor composition that is in equilibrium with the liquid composition at a fixed pressure or a fixed temperature. At equilibrium, the concentration of any component present in the liquid mixture is related to its concentration in the vapor phase by the equilibrium constant, Ki:
![]()
(6.1)
where yi is the mole fraction of component i in the vapor phase and xi the mole fraction of component i in the liquid phase. The more volatile components in a mixture will have the higher Ki values, whereas less volatile components will have lower values of Ki. The key separation factor in distillation is the relative volatility, defined as
![]()
(6.2)
The higher the value of the relative volatility, the more easily components may be separated by distillation.
In ideal systems the behavior of vapor and liquid mixtures obeys Dalton’s and Raoult’s laws. Dalton’s law relates the concentration of a component present in an ideal gas or vapor mixture to its partial pressure:
![]()
(6.3)
where pi is the partial pressure of component i in the vapor mixture, and P is the total pressure of the system given by the sum of the partial pressures of all components in the system:
![]()
(6.4)
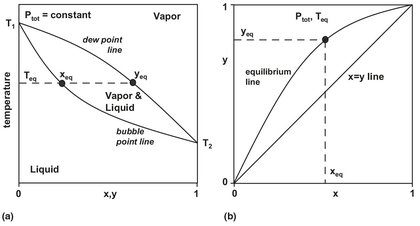
Fig. 6.3: Isobaric vapor-liquid equilibrium diagrams: (a) dew and bubble point; (b) y-x diagram.
Raoult’s law relates the partial pressure of a component in the vapor phase to its concentration in the liquid phase:
![]()
(6.5)
where ![]() is the vapor pressure of pure component i at the system temperature. Combining equations (6.3) and (6.5) yields
is the vapor pressure of pure component i at the system temperature. Combining equations (6.3) and (6.5) yields
![]()
(6.6)
This results in the following relation between the pure component vapor pressure, its equilibrium coefficient and the relative volatility for an ideal mixture:
![]()
(6.7)
Eq. (6.7) shows that for ideal systems the relative volatility is independent of pressure and composition. Vapor pressures for many components have been published in the literature and often correlated as a function of temperature by the Antoine equation:
![]()
(6.8)
where Ai, Bi, and Ci are Antoine constants, and T is the temperature in degrees Celsius or Kelvin. Since vapor pressures of components depend on temperature, equilibrium ratios are a function of temperature as well. However, the relative volatility is considerably less sensitive to temperature changes, because it is proportional to the ratio of the vapor pressures. In general the vapor pressure of the more volatile component tends to increase at a slower rate with increasing temperature than the less volatile component. Therefore, the relative volatility generally decreases with increasing temperature and increases with decreasing temperature. For a binary system the relative volatility equation can be rearranged to give
![]()
(6.9)
Eq. (6.9) is used to express the concentration of a component in the vapor as a function of its concentration in the liquid and relative volatility. It is plotted in Fig. 6.4 for various values of relative volatility. When relative volatility increases, the concentration of the most volatile component in the vapor increases. When the relative volatility is equal to 1, the concentrations of the most volatile component in the liquid and vapor phases are equal, and a vapor/liquid separation is not feasible.
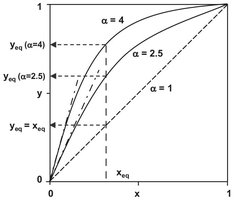
Fig. 6.4: Vapor-liquid equilibrium compositions as a function of relative volatility.
Most liquid mixtures are nonideal and require Raoult’s law to be modified by including a correction factor called the liquid phase activity coefficient:
![]()
(6.10)
Although at high pressures, the vapor phase may also depart ideal vapor mixture, the common approach in distillation is to assume ideal vapor behavior and to correct nonideal liquid behavior with the liquid phase activity coefficient. The standard state for reference for the liquid phase activity coefficient is commonly chosen as yi → 1 for the pure component. The liquid phase activity coefficient is strongly dependent upon the composition of the mixture. Positive deviations from ideality (yi > 1) are more common when the molecules of different compounds are dissimilar and exhibit repulsive forces. Negative deviations (yi < 1) occur when there are attractive forces between different compounds that do not occur for either component alone. For nonideal systems the relations for the distribution coefficient and relative volatility now become
![]()
(6.11)
In nonideal systems the distribution coefficients and relative volatility are dependent on composition because of the composition dependence of the activity coefficients. When the activity coefficient of a specific component becomes high enough, an azeotrope may be encountered, meaning that the vapor and liquid compositions are equal and the components cannot be separated by conventional distillation. Fig. 6.5 shows binary vapor-liquid composition (x-y), temperature-composition (t-x), and pressure-composition (P-x) diagrams for intermediate boiling, minimum azeotrope, and maximum azeotrope systems. A minimum azeotrope boils at a lower temperature than each of the components in their pure states. When separating the components of this type of system by distillation, such as ethanol-water, the overhead product is the azeotrope. A maximum-boiling azeotrope boils higher than either component in their pure states and is the bottom product of distillation. An example of this type of system is acetone-chloroform.
6.1.3 Separation by single-stage partial evaporation
Two main modes are utilized for single-stage separation by partial evaporation. The most elementary form is differential distillation, in which a liquid is charged to the still pot and heated to boiling. As illustrated by Fig. 6.6, the method has a strong resemblance with laboratory distillation, shown previously in Fig. 6.1. The vapor formed is continuously removed and condensed to produce a distillate. Usually the vapor leaving the still pot with composition yD is assumed to be in equilibrium with perfectly mixed liquid in the still at any instant. The distillate is richer in the more volatile components, and the residual bottoms are richer in the less volatile components. As the distillation proceeds, the relative amount of volatile components composition of the initial charge and distillate decrease with time. Because the produced vapor is totally condensed, yD = xD, there is only one single equilibrium stage, the still pot. Simple differential distillation is not widely used in industry, except for the processing of high-value chemicals in small production quantities or for distillations requiring regular sanitation.
The continuous form of simple single-stage equilibrium distillations is called flash distillation. A flash is a single equilibrium stage distillation in which a continuous feed is partially vaporized to give a vapor richer in the more volatile components than the remaining liquid. In Fig. 6.7, a liquid feed is heated under pressure and flashed adiabatically across a valve to a lower pressure, resulting in the creation of a vapor phase that is separated from the remaining liquid in a flash drum. If the equipment is properly designed, the liquid and vapor leaving the flash drum are considered to be in equilibrium. Mechanically a demister is used to prevent droplets from leaving with the vapor. Modeling is done through simple mass balances combined with equilibrium data or equivalent expressions. The overall mass balance is
![]()
(6.12)
and the component i balance becomes
![]()
(6.13)
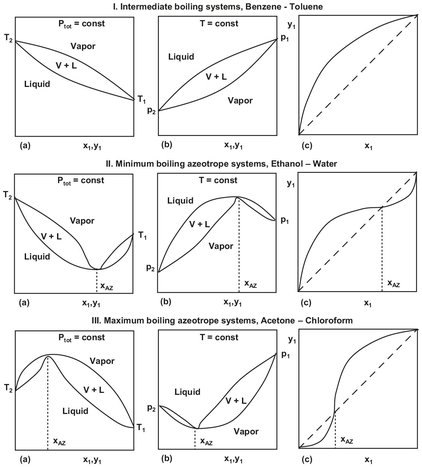
Fig. 6.5: Types of binary temperature-composition (a), pressure-composition (b), and x-y (c) phase diagrams for vapor-liquid equilbrium.
Unless the relative volatility is very large, the degree of separation achievable between two components in a single equilibrium stage is poor. Therefore, flashing is an auxiliary operation used to prepare streams for further processing and/or where a crude separation is adequate. Typically, the vapor phase is sent to a vapor separation system, while the liquid is sent to a liquid separation system.
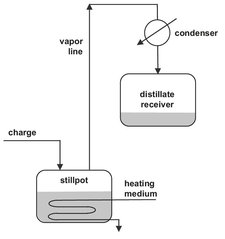
Fig. 6.6: Simple differential distillation.
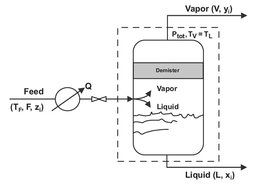
Fig. 6.7: Flash distillation.