Process Technology: An Introduction - Haan A.B. 2015
12 Membrane separations
12.1 Introduction
As a method of separation, membrane processes are rather new. Although membranes were already applied for the filtration of drinking water samples at the end of World War II, the breakthrough as an industrial separation process took off only after the discovery of the Loeb-Sourirajan process for making defect-free, high flux, asymmetric reverse osmosis membranes in the early 1960s. As illustrated in Fig. 12.1, the membrane splits a feed stream into a permeate stream, enriched in particles capable of permeating the membrane, and a retentate or concentrate stream that leaves the module without passing through the membrane. Although there are many membrane processes, based on different separation principles or mechanisms, they are all characterized by the use of a membrane to accomplish a particular separation. Every application exploits the ability of a membrane to control the permeation of a chemical species in contact with it. Separation is achieved because the membrane transports one component from the feed mixture more readily than any other component or components. Transport through the membrane takes place as result of a driving force acting on the components in the feed.
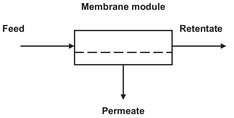
Fig. 12.1: Basic diagram of a membrane process.
This is schematically represented in Fig. 12.2. Phase 1 is usually considered as the feed or upstream phase, while phase 2 is considered as the permeate or downstream side. In many cases the permeation rate (N) through the membrane is proportional to the driving force across the membrane (ΔΧ):
![]()
(12.1)
where k is the proportionality coefficient. Driving forces can be gradients in pressure, concentration, electrical potential, or temperature.
An overview of the most important membrane processes and driving forces is given in Tab. 12.1. Well-established industrial membrane separation processes are microfiltration, ultrafiltration, nanofiltration, and reverse osmosis. The range of application of these four pressure driven membrane water separation processes is summarized in Fig. 12.3. Both, ultrafiltration and microfiltration employ sieving through increasingly fine pores as the mode of separation. Microfiltration membranes filter colloidal particles and bacteria from 0.1 to 10 µm in diameter. Ultrafiltration membranes can be used to filter dissolved macromolecules, such as proteins, from solutions. The mechanism of separation by reverse osmosis membranes is quite different. In reverse osmosis the membrane pores are so small that they are within the range of thermal motion of the polymer chains that form the membrane. Nanofiltration membranes fall into a transition region between pure reverse osmosis and pure ultrafiltration. Both techniques are used when low molecular weight solutes, such as inorganic salts or small organic molecules, have to be separated from a solvent. The most important application of reverse osmosis is the desalination of brackish groundwater or seawater.
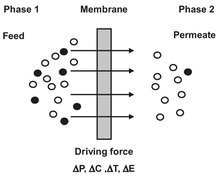
Fig. 12.2: Schematic representation of selective membrane permeation.
Tab. 12.1: Most important industrial membrane processes.
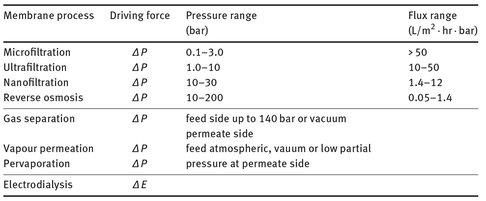
In gas separation and vapor permeation, a gas mixture is passed across the surface of a membrane that is selectively permeable to one component of the feed mixture. Major current applications of gas permeation include the separation of hydrogen from nitrogen, argon and methane in ammonia plants, the production of nitrogen from air, and the separation of carbon dioxide from methane in natural gas applications. Pervaporation is the only membrane process where a phase transition occurs. A liquid mixture contacts one side of a membrane, and the permeate is removed as a vapor from the other side. The driving force for the process is the low vapor pressure on the permeate side of the membrane. Currently the main industrial application of pervaporation is in the dehydration of organic solvents. Electrodialysis applies charged membranes to separate ions from aqueous solutions under the driving force of an electrical potential difference. The principal application is the desalting of brackish groundwater. However, other industrial applications such as pollution control are rapidly growing.
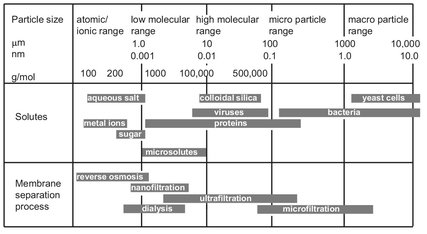
Fig. 12.3: Application range of various membrane processes.
Membrane processes possess a number of distinct advantages compared to alternative separation methods such as distillation, absorption, and extraction. The main advantage is that most membrane separations consume less energy, because generally no phase transition is involved. Additional advantages are that the separation can be carried out under mild conditions, and scale-up is easily accomplished by simply increasing the membrane area. However, scaling-up by applying more modules in parallel makes the investment costs of a membrane separation process increase almost linearly with scale, which becomes a disadvantage at higher capacities. Additional limitations of membrane processes are limited number of stages requiring a high selectivity for a given separation and the chemical/thermal stability of the membrane material. Also fouling of membranes may restrict permeability and/or lifetime of membranes and even make them unsuited for some types of feed streams.