Process Technology: An Introduction - Haan A.B. 2015
2 The structure of chemical and biochemical process systems
2.2 Characteristics of production processes
The practical way a product is prepared on a laboratory scale is in many ways different from the way it is done on an industrial scale. The main reasons for this are the huge differences in the amount of material that has to be processed. On a laboratory scale one is usually satisfied with amounts that are sufficient for analytical purposes. Industrial production concerns the production of amounts that can vary from 1000 kg/year up to over 1000 000 000 kg/year for a single plant. It is clear that such large amounts should have significant effects on the way a production process is designed.
2.2.1 Batch production technology
Batch processing has been a part of human activities throughout history and is still used most of the time on a laboratory scale. Batch processes are used to manufacture many of the products required for modern life. Within the chemical process industries, batch processing is focused on the fine and speciality chemicals sectors, while continuous processing is dominant in commodity chemicals production.
A batch process is one in which a series of operations are carried out over a period of time on a separate, identifiable item or parcel of material. It is different from a continuous process, during which all operations occur at the same time and the material being processed is not divided into identifiable portions. This definition of batch processing includes what has been called semibatch production, during which material is added continuously to a batch over some period. The sequence of events copies the sequence developed in the laboratory, but in larger-size vessels and batches. The raw materials are purified, perhaps by distillation or adsorption, and stored. Reactants are then pumped or poured into a reaction vessel. The agitation intensity, the rate of heating and/or cooling, and the rates of flow of other reactants or catalysts are controlled in such a manner that the reaction proceeds as planned. When the reaction is completed, the reactant mass is removed to a separation system. The desired products are separated from unreacted feed materials and undesired byproducts. The reactants are usually recycled for use in the next batch. This is schematically represented in Fig. 2.2.
Batch processing is typically applied for small-volume products and in cases where the fundamental mechanisms of the reaction are not well known. This issue of robustness to incomplete knowledge is of extreme importance for the production of fine chemicals in a multi-purpose environment. Batch processes often use complex chemistry of which substantially less is known than a typical continuous process. The cost of evaluating kinetics and physical parameters to the accuracy required for using standard chemical engineering design methods would be far too high. However, such detailed analysis is seldom necessary. It is usually sufficient to know how long an operation will take, and even that need not be known to excessive accuracy. The use of rules of thumb for scale-up based on identification of the rate-controlling process is a common way of assessing the time taken for an operation on the full scale. Discrepancies between the expected and actual times for each operation tend to average out over the many operations that constitute a single batch. Thus, the impact of inaccurate knowledge is much less serious than for a continuous process whose overall productivity is limited by the capacity of whichever unit has lowest throughput.
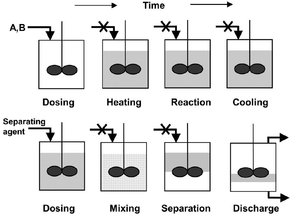
Fig. 2.2: Schematic representation of a batch process operation.
Besides robustness, batch production plants offer us the second advantage of extreme flexibility. In general the plant used, typically employing stirred vessels of either stainless or glass-lined steel of 1 ~ 50 m3 in volume, is easily modified for use on new products. Such equipment is very versatile and can be used to blend reactants, heat them to reaction temperature, carry out the reaction, cool, distil off solvent, and crystallize the product. In a continuous process, each operation would be carried out in a separate unit.
Small-scale processes and processes in which solids occur are likely to be batch. Numerous chemical process industries retain batch processing as their primary method of manufacture. Many of these continue to be made batchwise because quality is more important than price. The product has traditionally been made so, or the industry is not large enought or technically sophisticated enough to operate successfully in a continuous mode. Typical products manufactured by batch processes include pharmaceuticals, agrochemicals, dyestuffs, photosensitive materials, food additives, perfumes, vitamins, pigments, and many more.
2.2.2 Continuous processes
For large production capacities where the process reaction mechanism is better known and reaction rates are not too slow, continuous processing is often possible. Here, the raw materials are prepared and continuously fed to the reactor. The reactor system is sized so that the materials reside in it long enough at the reaction conditions to achieve the desired extent of reaction. The reaction system may be a single vessel or a number of reactor vessels in series, each operating under different conditions. The product continuously leaves the reaction zone and passes to a sequence of separation steps where the desired products are obtained in continuous streams. Unreacted feed materials are obtained in other streams and continuously returned to the reactor. Any by-products are also removed.
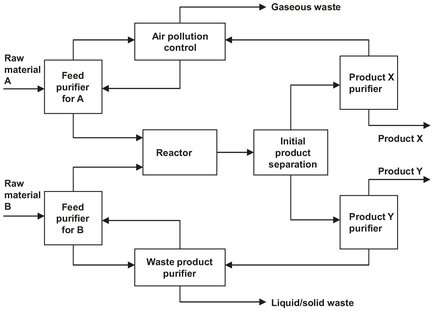
Fig. 2.3: Typical continuous process plant.
Fig. 2.3 shows a possible configuration for a typical continuous process plant. Two raw materials are fed to a reactor after each is purified. The reactor effluent is separated in three separation steps, and all waste streams are treated before release to the environment. Product X might be the main product desired and product Y a salable byproduct. Ideally, such a process operates under steady-state conditions, i.e. a stable operating condition where none of the process parameters (temperature, pressure, process stream composition, flow rate, etc.) vary with time. In any real process there will be a period of adjustment as the plant is started or stopped, and there will be disturbances as the process operates. The process control system attempts to minimize the effects of these process upsets.
In a plant operating under steady-state conditions, variables are different from point to point along the process path. But if you were to take a photograph of the control panel, where an array of instruments records the process conditions at all the crucial points in the process, you would see no differences between a picture taken at 8 a.m. and one taken at 8 p.m. Some basically continuous processes have components, for example, dryers, filters and ion exchange beds that operate cyclically. These units must be taken off-stream periodically for regeneration. The period of on-stream operation may be several hours to several days.
Most large-scale processes operate continuously, especially ones in which gases and low-viscosity liquids are handled. Petroleum refining, the manufacture of bulk chemicals, and industrial gas manufacturing are typical examples.
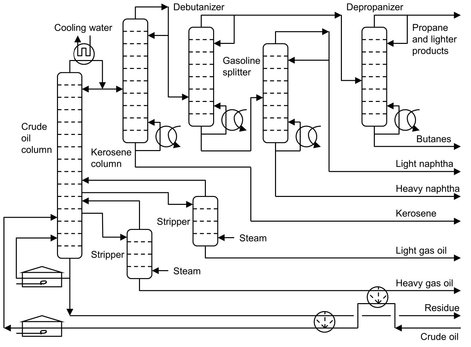
Fig. 2.4: Flow chart of an atmospheric crude oil distillation train.