Process Technology: An Introduction - Haan A.B. 2015
14 Solids finishing technologies
14.2 Drying
Drying is an operation in which volatile compounds (usually liquids) are removed from solids, slurries, and solutions, to yield solid products. The separation may be carried out in a mechanical manner without phase change or by evaporation through the supply of heat. Thermal drying consists of two steps. First heat is supplied to the material to evaporate the moisture out of the product. Secondly, the vapor is separated from the product phase and, if necessary, condensed outside of the dryer. This thermal drying is discussed in the following sections. If possible, mechanical predrying is installed upstream of thermal drying because solids handling is made easier and liquid separation without evaporation is less costly (Fig. 14.2).
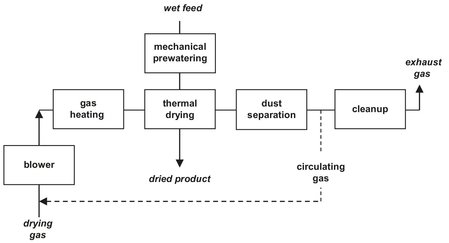
Fig. 14.2: Open and partial air recirculation (---) convective drying system.
The goal of most drying operations is not only to separate a volatile liquid, but also to produce a dry solid of a desirable size, shape, porosity, density, texture, color, or flavor. Drying is usually the final processing step before packaging and makes many materials, such as soap powders and dyestuffs, more suitable for handling. Drying or dehydration of biological materials, especially foods and biopharmaceuticals, is used as a preservation technique. The product that is to be dried is denoted as the moist solid. In most cases the liquid being removed is water, but it could also be a solvent such as alcohol or hexane. The substance that carries the necessary heat is called the drying agent. This substance can be air, an inert gas such as nitrogen, or superheated steam. Radiation, hot surfaces, or microwaves can also be used to supply the required heat. Because all drying operations involve processing of solids, equipment material handling capability is of primary importance. In fact, most industrial dryers are derived from material handling equipment designed to accommodate specific forms of solids. Environmental factors, such as emission control and energy efficiency, increasingly influence equipment choices.
14.2.1 Classification of drying operations
Drying methods and processes can be classified in several ways. One classification is batch, where the material is inserted into the drying equipment and drying proceeds for a given period of time, or continuous drying, where the material is continuously added to the dryer and dried material continuously removed. A batch dryer is best suited for small lots and for use in multiple-product plants. This dryer is one into which a charge is placed, the dryer runs through its cycle, and the charge is removed. In contrast, continuous dryers operate best under steady-state conditions, drying continuous feed and product streams. Optimum operation of most continuous dryers is at design rate and steady-state. Continuous dryers are unsuitable for short operating runs in multiproduct plants.
Drying processes can also be classified according to the physical conditions used to add heat and remove water vapor. The most frequently applied heat transfer mechanisms are convection drying and contact drying. In convection drying the sensitive heat of a hot gas is supplied to the material surface by convection. The drying agent flowing past or through the body also removes the evaporated water and transports it from the dryer (Fig. 14.2). To save energy, partial recirculation of the drying medium is also used. Drying operations involving toxic, noxious, or flammable vapors employing gas-tight equipment combined with recirculating inert gas systems which have integral dust collectors, vapor condensers, and gas re-heaters (Fig. 14.3). In contact drying the heat is supplied to the wet material by conduction from the heated surface as bands, plates, cylinders, or the dryer wall. The amount of heat transferred depends not only on the thermal conductivity of the heating surface but also on the heat transfer coefficient from the heating medium to the surface. Common heating mediums include steam, organic liquid, and molten metals. Since all the heat for moisture evaporation passes through the material layer, the thermal efficiency of contact drying is higher than convective drying, where most of the heat is flowing over the material and wasted into the outlet air.
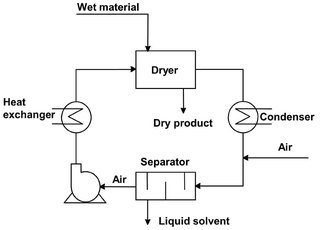
Fig. 14.3: Convective drying in a closed system.
14.2.2 Drying mechanisms
In the design of drying operations an understanding of liquid and vapor mass transfer mechanisms is essential for quality control. Because no two materials behave alike, this understanding is usually obtained by measuring drying behavior under controlled conditions in a prototypic, pilot-plant dryer. From these experiments the drying rate is usually determined as the change of moisture content with time. For most moist solids, especially those having capillary porosity, the drying rate depends upon the moisture content in a manner similar to that shown in Figs. 14.4 and 14.5. In the initial drying period the solid and the liquid have a lower temperature and need to be raised to its ultimate drying temperature by the hot gas stream. This initial heating period is usually quite short and can often be ignored.
After reaching the equilibrium temperature the drying rate remains practically constant for a period of time. During this period the surface of the solid is initially very wet, and a continuous film of water exists on the drying surface. This water is entirely unbound and acts as if the solid were not present. Under these conditions the rate of evaporation is independent of the solid and is essentially the same as the rate from a free-liquid surface. The evaporation rate is controlled by the heat transfer rate to the wet surface. The mass transfer rate adjusts to the heat transfer rate, and the wet surface reaches a steady-state temperature. This temperature is called the wet bulb temperature and indicates the maximum weight of vapor that can be carried by an amount of dry gas. As a result the drying rate remains constant so long as the external conditions are constant. Under these conditions all principles relating to simultaneous heat and mass transfer between gases and liquids apply. The steady state drying rate dw/dt (kg/s) can be obtained from the conditions that the heat flux from the drying agent to the solid is equal to the product of the drying rate and the vaporization enthalpy ΔHv:
![]()
(14.1)
If the solid is porous, most of the water which is evaporated in the constant rate period is supplied from the interior of the solid. The constant rate period is continuous only as long as the water is supplied to the surface as fast as it is evaporated. During constant rate drying, the material temperature is controlled more easily in a direct-heat dryer than in an indirect-heat dryer, because in the former drying process the material temperature does not exceed the gas wet bulb temperature as long as all surfaces are wet.
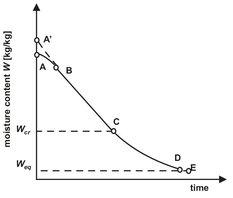
Fig. 14.4: Drying curve.
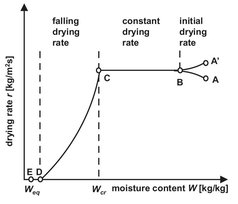
Fig. 14.5: Drying rate curve.
When the moisture content is reduced below a critical value, the surface of the solid dries out, and further evaporation starts taking place in the interior of the porous solid. This is called the falling rate drying period. First the wetted surface area decreases continuously until the surface is completely dry, and the plane of evaporation slowly recedes from the surface. Heat for evaporation is transferred through the solid to the zone of evaporation. The drying rate is controlled by the internal material moisture transport and decreases with decreasing moisture content. The amount of moisture removed in the falling rate period may be relatively small, but the required time is usually long. At the end of the falling rate period the remaining moisture in the solid is bound by sorption. The drying rate decreases rapidly with decreasing moisture content and tends to zero as the hygroscopic equilibrium moisture content is approached.
This equilibrium moisture content is the steady-state equilibrium reached by the gain or loss of moisture when material is exposed to an environment of specific temperature and humidity for a sufficient time. The equilibrium state (Fig. 14.6) is independent of the drying method or rate. It is a material property that relates to the moisture partial vapor pressure in the drying gas p by Henry’s law:
![]()
(14.2)
where K is Henry’s constant and x is the equilibrium dry basis moisture content. From this relation it is clear that low residual moisture contents are obtained at low partial pressures in the drying gas. This is usually achieved by removing the moisture from the drying gas at a low temperature and heating the gas before drying. Instead of using the moisture partial pressure the equilibrium moisture content can also be related to the relative humidity of the drying air, which is defined as
![]()
(14.3)
where p* is the moisture saturation pressure at the operating temperature. Introduction in eq. (14.2) results in an expression where an alternative Henry’s constant K* is used that is almost independent of temperature:
![]()
(14.4)
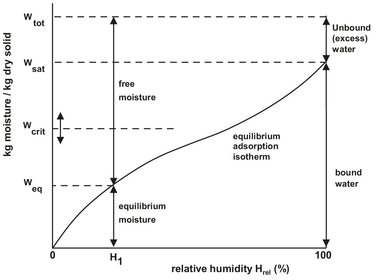
Fig. 14.6: Graphical illustration of the various kinds of moisture.
The saturation temperature curve indicates the maximum weight of vapor that can be carried by a unit weight of dry gas. At saturation the partial pressure of vapor in the gas equals the vapor pressure of the liquid at the specific temperature:
![]()
(14.5)
where HS is the saturation humidity, the weight ratio of moisture/kg dry gas, P is the total system pressure, and MV/MG the molecular weight ratio of vapor to dry gas. At any condition less than saturation, the humidity H is expressed similarly:
![]()
(14.6)
14.2.3 Direct-heat dryers
In direct-heat dryers, steam-heated, extended surface coils are used to heat the drying gas up to temperatures as high as 200 °C. Electric and hot oil heaters are used for higher temperatures. Diluted combustion products are suitable for all temperatures.
In most direct-heat dryers, more gas is needed to transport heat than to purge vapor. Some of the most commonly used direct-heat dryers are listed below.
Batch compartment dryers
Direct heat batch compartment dryers are often called tray dryers because of frequent use for drying materials loaded in trays on trucks or shelves. Fig. 14.7 illustrates a two-truck dryer. The compartment enclosure comprises insulated panels designed to limit exterior surface temperature to less than 50 °C. Slurries, filter cakes, and particulate solids are placed in stacks of trays. Large objects are placed on shelves or stacked in piles. Unless the material is dusty, gas is recirculated through an internal heater as shown. Only enough purge is exchanged so as to maintain the needed internal humidity. For inert gas operation, purge gas is sent through an external dehumidifier and returned. These dryers are economical only for single-product rates less than 500 t/yr, multiple product operation, and batch processing. Variable speed fans are employed to provide higher gas velocity over the material during early drying stages. To minimize dusting, the fans reduce velocity after constant drying when heat transfer at the material surface is no longer the limiting drying mechanism. Shallow tray loading yields faster drying, but care is needed to ensure depth uniformity and labor is increased.
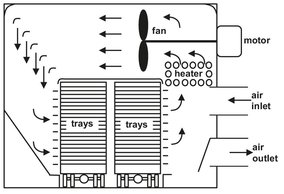
Fig. 14.7: Two-truck tray dryer. Adapted from [1].
Belt dryers
In belt dryers a loading device especially designed for the product is used to place the moist solid on the surface of a circulating belt, which passes through a drying chamber resembling a tunnel (Fig. 14.8). The solid remains undisturbed while it dries. At the end of this chamber the material falls from the belt into a chute for further processing. In some installations the material falls onto another belt that moves in the opposite direction to the first one. Centrifugal or axial flow blowers are used to aerate the moist materials. The air stream can enter the solid from below or above. Belt dryers are ideal for friable, molded, granular, or crystalline products that require a long, undisturbed drying time. They are used in all branches of industry.
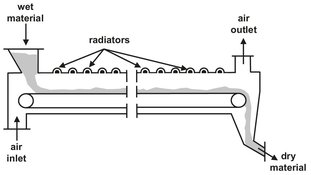
Fig. 14.8: Radiation belt dryer.
Rotary dryers
A direct-heat rotary dryer is a horizontal rotating cylinder through which gas is blown to dry material that is showered inside (Fig. 14.9). Shell diameters are 0.5-6 m. Batch rotary dryers are usually one or two diameters long. Continuous dryers are at least four and sometimes ten diameters long. At each end, a stationary hood is joined to the cylinder by a rotating seal. These hoods carry the inlet and exit gas connections and the feed and product conveyors. For continuous drying the cylinder may be slightly inclined to the horizontal to control material flow. An array of material showering flights of various shapes is attached to the inside of the cylinder. The dry product may be recycled for feed conditioning if the material is too fluid or sticky initially for adequate showering. Slurries may also be sprayed into the shell in a manner so that the feed strikes and mixes with a moving bed of dry particles. Material fillage in a continuous dryer is 10-18 % of the cylinder volume. Greater fillage is not showered properly and tends to flush towards the discharge end.
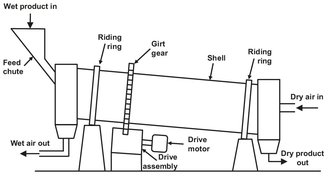
Fig. 14.9: Direct heat rotary dryer.
Direct-heat rotary dryers are the workhorses of bulk solids industries. Most particulate materials can somehow be processed through them. These dryers provide reasonably good gas contacting, positive material conveying without serious back-mixing, good thermal efficiency, and good flexibility for control of gas velocity and material residence time. Gas flow in these rotary dryers may be cocurrent or counter-current. Cocurrent operation is preferred for heat-sensitive materials, because gas and product leave at the same temperature. Countercurrent allows a product temperature higher than the exit gas temperature, which increases the dryer efficiency. To prevent dust and vapor escape at the cylinder seals, most rotary dryers operate at a slightly negative internal pressure.
Flash dryers
In flash dryers materials are simultaneously transported and dried. The simplest form, illustrated by Fig. 14.10, consists of a vertical tube in which granular or pulverized materials are dried while suspended in a gas or air stream. The available drying time is only a few seconds. Only fine materials with high rates of heat and mass transfer or coarser products with only surface moisture to be removed are used in such dryers. Solids that contain internal moisture can only be dried to a limited extent by this method. Flash dryers are well suited for drying thermally sensitive materials and are widely used for drying organic and inorganic salts, plastic powders, granules, foodstuffs, etc.
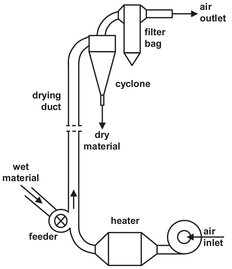
Fig. 14.10: Flash dryer.
Fluidized bed dryers
These units are known for their high drying efficiency. A two-stage model is illustrated in Fig. 14.11. The solid moves horizontally in a chute and the drying agent flows vertically through a perforated floor to fluidize the solid. These machines can operate continuously, because the solid is transported while suspended in the drying agent. Materials that can be suspended in the drying agent are usually powders, crystals, and granular or short-fibred products that remain finely divided. Pastes and slurries are mixed with previously dried material and are then easily fluidized.
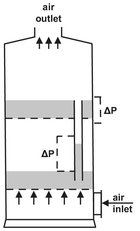
Fig. 14.11: Two-stage fluid bed dryer.
Spray dryers
Spray drying is used for the drying of pastes, suspensions, or solutions. The moist material is sprayed into the drying agent and converted into a powder that is entrained by the gas stream. A spray dryer is a large, usually vertical chamber through which hot gas is blown and into which a solution, slurry, or pumpable paste is sprayed by a suitable atomizer. The largest spray-dried particle is about 1 mm, the smallest about 5 µm. Because all drops must attain a nonsticky state before hitting the chamber wall, the largest drop produced determines the size of the drying chamber. The chamber shape is determined by the nozzle or disk spray pattern. Nozzle chambers are tall towers, usually having height/diameter ratios of 4: 5. Disk chambers are of large diameter and short. A spray dryer may be cocurrent, counter-current, or mixed flow. Cocurrent dryers are used for heat-sensitive materials. Counter-current spray dryers yield higher bulk density products and minimize production of hollow particles. Fig. 14.12 shows an open-cycle, cocurrent disk atomizer chamber with a pneumatic conveyor following for product cooling. Spray dryers are often followed by fluid beds for second-stage drying or fine agglomeration. Spray dryers are particularly suitable for drying solids that are temperature sensitive. Applications include coffee and milk powders, detergents, instant foods, pigments, dyes, etc.
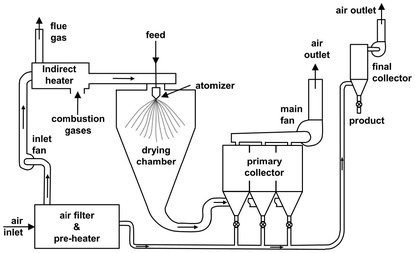
Fig. 14.12: Spray dryer system. Adapted from [1].
14.2.4 Contact dryers
In contact dryers most of the heat is transferred by conduction. Common heating media are steam and hot water, depending on the actual operating temperature. Based on dryer costs alone, contact dryers are more expensive to build and install than direct heat dryers, but they avoid the need of expensive air treatment units. For environmental concerns contact dryers are more attractive because they are more energy efficient and use only small amounts of purge gas. Dust and vapor recovery systems for contact dryers are smaller and less costly. In this paragraph some of the commonly encountered contact dryers are described.
Rotary and agitator dryers
The heat necessary for drying is transferred through the peripheral walls of these dryers in contact drying. Only a small amount of air is necessary to carry off the moisture that is taken from the solid. Accordingly, the air velocity in these units is quite low. This is advantageous when drying materials that dust easily or form dust during drying. The rotary steam tube dryer is a horizontal rotating cylinder in which one or more circumferential rows of steam-heated tubes are installed. In agitator dryers (Fig. 14.13) the vessel is stationary, and the solids product is slowly agitated. Material holdup may be varied from a few minutes to several hours. Agitator speeds rarely exceed 10 min-1, because at higher speeds the mechanical stress and power demand become unacceptable.
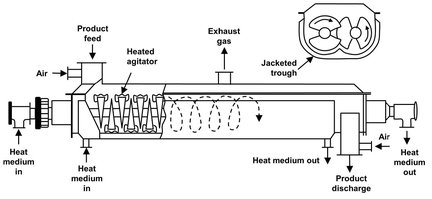
Fig. 14.13: Indirect heat paddle-type agitator dryer.
Vacuum dryers
The principal differences in the vacuum dryers are their seals and the means to produce the vacuum. Drying under reduced pressure is advantageous for materials that are temperature sensitive or easily decomposed, because the evaporation temperature is reduced. Vacuum dryers are most often used to dry pharmaceutical products and foodstuffs. The simplest form of a vacuum dryer for batch drying is the vacuum shelf dryer. The moist solid lies on a heated plate. Improved heat transfer with higher efficiency is obtained in the vacuum tumble dryer (Fig. 14.14), in which the moist solid is constantly agitated and mixed. These tumble dryers are also used for the final drying step during the production of various polyamides.
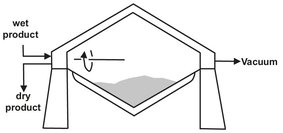
Fig. 14.14: Tumble vacuum dryer.
Fluidized bed dryers
Indirect-heat fluidized bed dryers are usually rectangular vessels in which vertical pipe or plate coils are installed. Fig. 14.15 is diagram of a two-stage indirect-heat fluid bed incorporating pipe or plate coils heaters. The general design is used for several particulate polymers. Excellent heat transfer is obtained in an environment of intense particle agitation and mixing. Because of its favorable heat— and mass-transfer capabilities, flexibility for staging, and lack of rotary seals, an indirect-heat fluid bed is an ideal vessel for vapor recovery and drying in special atmospheres.
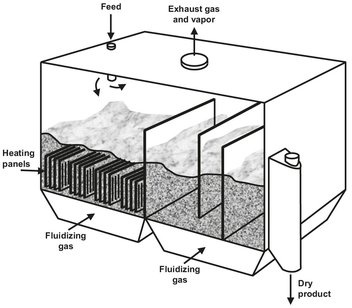
Fig. 14.15: Indirect heat fluid bed dryer.
14.2.5 Other drying methods
Some other, less frequently used drying methods are radiation heating, dielectric drying, and freeze drying. In radiation heating the energy is supplied from an electromagnetic radiation source remote from the surface of the solid. The electromagnetic radiation energy is absorbed by vibrations of molecules in the wet product, creating a thermal effect and evaporating moisture. Radiation drying is expensive because of the high cost of electrical power or combustion gas to heat the radiators. Since the penetration depth of infrared waves is relatively small, it is mainly used for short drying times of thin product layers such as films, coatings, and paints.
With high frequency or dielectric drying, the wet product forms a dielectric between the electrodes of a plate capacitor exposed to a high frequency electric field. The generated intermolecular friction causes the generation of heat inside the product. This is used to heat up the product and to evaporate the moisture. High frequency drying allows gentle thermal drying of the product. Substantial deformation and shrinkage cracks are avoided. Therefore, dielectric drying is employed for gentle drying of products such as fine wood, ceramic products, foods, pharmaceuticals, and luxury goods.
Freeze drying is a vacuum sublimation drying process. At temperatures below 0 °C and under vacuum, moisture sublimes from a frozen wet product directly from the solid to the gaseous state. Firstly the wet product has to be frozen from -15 to approximately -50 °C. The freezing rate and final temperature essentially determine the drying time and final quality of the product such as structure, consistency, color, and flavor. The frozen product is usually granulated, sieved, and then charged to the dryer. Under vacuum conditions it is then dried either discontinuously on heated plates or continuously while mixed and moved over a heated surface. Sublimed moisture vapor solidifies to ice on a cooling agent operated condenser. Drying rates are low because the low allowable rate of heat flow controls the process. Freeze or sublimation drying is carried out in a chamber freezer, vacuum disc dryer, vibrating film dryer, cascade dryer, or spray freezing dryer. The high investment and operating costs of freeze drying are only worthwhile for high-grade, thermally sensitive products. Certain important properties of the products such as flavor, taste, color, and protein conformation are retained, so that biologically active proteins maintain their biological activity (e.g. vaccines).