Process Technology: An Introduction - Haan A.B. 2015
15 Product technology
15.1 Cheese-coating technology
15.1.1 Cheese production
The raw material for cheese manufacture is fresh raw milk. Most production techniques are intended to selectively increase the casein, fat, and dry matter content in cheese by expelling whey. Moreover, they create appropriate conditions that permit lactose to lactate fermentation and ripening to develop in a way that is specific to each type of cheese. The starter cultures used for lacate fermentation consist of lactic acid bacteria and are introduced in amounts ranging from 0.05 to 5 wt%. Cheese yield depends primarily on the fat and protein content of the milk. The cheese-making process begins with coagulation of the milk in a vessel. The salt and protein concentration as well as the acidity are very important for coagulation. Rennet enzymes, acid, or combinations of enzymes and acid coagulate the casein, and the resulting casein particles form a three-dimensional network enclosing the other milk components. As soon as the coagulum has attained the desired consistency, it is cut into cubes. In this curdmaking process, the solid curds are separated from the liquid whey containing the dissolved lactose, whey protein, and salts. Whey expulsion is subsequently regulated according to the final water content desired in the ripe cheese. When the curd is firm enough, it is separated from the whey by transferring to a perforated metal or plastic mold. The next stage is the further expulsion of whey by pressing and turning the cheese in the mold. After salting, the cheese is submitted to further curing and ripening procedures. The characteristics of the end product are determined by lactic acid breakdown, proteolysis, and lipolysis. The principal cheese quality criteria are odor, taste, body texture, shape appearance, and in some cases eye formation.
Ripening takes place in special rooms where temperature, relative air humidity, air motion, and treatment of the cheese are essential factors. One of the most important points during ripening and storage of cheese is the prevention of mould and bacteria growth on the cheese. Moulds attack the cheese crust, which in turn becomes sensitive to attack by other parasites such as cheese mites and the product becomes unattractive to the eye of the customer. Furthermore moulds excrete components that have a negative effect on the taste of the cheese. Some of these components may even be toxic. Although many measures can be taken to minimize the growth of moulds, it appears very difficult in practice to keep the cheese completely free of mould growth. This has resulted in the use of so-called plastic coatings, which are applied to the cheese when it is drying. After drying the plastic-coating layer forms a strong barrier that prevents mould from taking nutrients from the cheese crust. Moreover, many of these coatings contain additives which prevent mould growth.
15.1.2 Coatings
Coating agents are compounds that are used to cover or coat foods which are added to food surfaces for their protection. Coatings are not a packaging material, and they are also not food additives, because coatings are not intended for consumption. Coating agents are used to protect foods or their surfaces against undesirable changes, such as drying out and loss of aroma. In some foods, coating agents may prevent oxidation or the growth of undesirable microorganisms on the surface. Other coating agents give an attractive look to the surface of a food that would not otherwise look very appealing. Coatings are applied in liquid, paste, or powder form, are applied to surfaces in layers of a given thickness, and form adherent films on the surface of the substrate. These coatings generally have a pronounced yellow, ochre, red, brown, or black color permitting the standardization of the visual display of marketed products.
For most uncooked pressed cheeses, treatments aim to limit or prevent the development of wild flora spontaneously settling on the surface of the cheese. An important technology to prevent the growth of wild flora is to apply, at the beginning of ripening, films of a varied nature (wax, paraffin wax, plastic film, varnish) by immersion or spraying. Polyvinyl acetate (PVA) coatings are useful for protecting high-quality hard and semihard naturally ripened cheeses against mechanical damage and mould growth. PVA is an emulsion of copolymers in water which enhances the natural ripening qualities of cheese and leads to optimum standardization in appearance and characteristics. The function of the coating inn ripening and storage is to protect the cheese from mechanical, physical, and microbial damage or spoilage, and ultimately to improve the final presentation and consumer appeal of the cheese. When cheese coatings are applied, it is important to achieve a uniform distribution of coating on the cheese, and the correct amount and distribution of the antimicrobial agent (natamycin) to protect against moulds and yeast growth both in the coating and on the cheese, as well as to allow the coating to dry properly before turning and subsequent treatments. As long as the film is intimately in contact with the cheese without discontinuities caused by the presence of air or residual whey, protection is efficient.
15.1.3 Application techniques
Many techniques have been developed for the industrial application of coatings. Analysis of the criteria for choosing a coating method is a complex task. Economic factors in relation to the performance targets generally have top priority for choosing an application method on a commercial basis. Coating systems and processes are usually preferred which best satisfy the demands and requirements for thin coatings, a high degree of material utilization, low energy costs, and good automation.
Dip and brush coating
Dip and brush coatings are among the oldest coating methods. In conventional dip coating (Fig. 15.1) the item is fully immersed in the coating fluid and then withdrawn. The liquid paint adheres to the surface and is then dried or heat cured. Care should be taken to ensure that the items do not float during dipping and that air bubbles do not become trapped. The speed at which the item is removed from the bath must be selected so that excess coating fluid adhering to the surface can run off. Draining and evaporation times must be sufficiently long to ensure satisfactory evaporation of the solvents (if necessary a hot air zone should be included for waterborne paints). A benefit of al dipping processes is that the items are completely coated with only low coating losses, and the processes are very easily automated. At the same time, however, variations in coating thickness and tearing cannot always be avoided, so requirements with respect of surface quality should not be too tight.
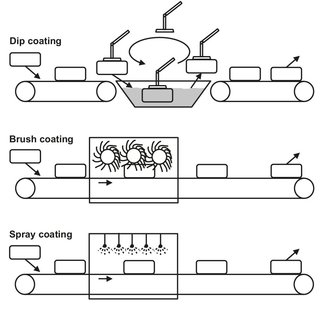
Fig. 15.1: Schematic of an automated dip (top), brush (middle) and spray (bottom) coating machine.
Application of a coating with a brush is commonly used for small parts and for retouching sections of surfaces. Advantages of brushing (Fig. 15.1) are the good wetting and covering of surface defects, low coating losses, and flexibility regarding shape. Disadvantages and limitations include the labor intensity, criticality to flow, and nonuniformity of the applied coating layer. The material and shape of the brush used should be selected according to the coating material and the object. For example, water-borne coatings and solvent-based coatings, and even high— and low-viscosity materials, each require a different brush.
Spraying (atomization)
In spraying of either liquids or powders, two fundamentally different methods are used: conventional spraying and electrostatic spraying. In conventional spraying (Fig. 15.1), compressed air flows through an annular gap in the head of the spray gun that is formed between a bore in the air cap and the concentric paint nozzle. Further air jets from air-cap bores regulate the jet shape and assist atomization. The expanding compressed air leaves the paint nozzle at high velocity. A low-pressure area is formed in the nozzle aperture which exerts a suction effect and assists outflow of the coating fluid. The difference between the velocities of the compressed air and the exiting paint atomizes the paint into particles that are conveyed as spherical droplets in the free jet. The size of the droplets produced depends on the pressure applied in the process. At relatively high pressures, 2—7 bar, the droplets can be as small as 10 µm in diameter, whereas a lower pressure results in droplet sizes of 20—300 µm.
Drying
Although the vast majority of coatings are applied in the fluid state, coatings are only functional when dry. Thus the drying process is just as important as the coating process itself, as it can either enhance or deteriorate the properties of the coating. The purpose of the drying process is to produce a uniform dry coating from the applied wet coating by evaporating inert solvent from the functional coating ingredients (polymer, binder, pigments, dyes, hardener, coating aids, and so forth), so that only the desired coating remains on the substrate. Solvents used in the coating process can range from water to volatile organic materials. Usually, thermal drying is used, where heat supplied to the fluid coating evaporates the solvent. If the coating is applied uniformly, then the dryer must immobilize it and maintain the uniformity of the coating throughout the drying process. However, some coatings are applied with nonuniformities and level out during the drying process, as in brush and spray coating. Solvent removal must be done without adversely affecting the coating formulation or interfering with the desired uniformity of the coating.
Film formation
Even though most emulsion paints contain pigments, fillers, and additives in addition to the polymer dispersion, only the role of the polymer dispersion in describing the film-forming process, schematically illustrated in Fig. 15.2, will be considered. When the water evaporates from the emulsion paint after application, the polymer particles move closer together. As soon as they touch each other, the water starts to evaporate from the space between them, and capillary forces begin to develop. The polymer particles are deformed by these capillary forces and congregate even more closely together. Upon further drying, increasingly large forces thus develop in the capillaries, which likewise become narrower. Under the resulting pressure the liquid is expelled and the polymer particles ultimately fuse.
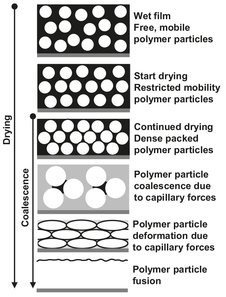
Fig. 15.2: Model representation of film forming in a polymer dispersion.