Process Technology: An Introduction - Haan A.B. 2015
15 Product technology
15.2 Enzyme formulation
15.2.1 Introduction
Industrial applications of enzymes form an important branch of biotechnology. Enzymes are biologically active catalysts which can induce very specific reactions under gentle conditions. They are used in the detergent industry and important industrial processes such as the conversion of starch to glucose syrups (α -amylase and amyloglucosidase) and the production of fructose syrups from glucose (glucose-isomerase). When an enzyme is recovered from a fermentation broth, it is usually present in an aqueous solution or processed to a dried state. Both types of products have to be formulated to comply with requirements appropriate to their final application. Requirements related to the storage of enzymes until the time of application include enzyme, microbial, and physical stability. Some applications demand special requirements such as having no precipitate, off-odor/taste, and off-color.
Any formulation is a compromise between the requirements just discussed. For example, the pH necessary for good microbial or physical stability may differ from the pH that gives an optimum enzyme stability. Product formulation is an important subject in the production of enzyme containing biotechnological products. It is the final and probably most essential step between production and application. There is a variety of formulation objectives such as dissolving, suspending, reuse, stabilization, conservation, dust prevention, and protection against an aggressive environment. As is the case for the encapsulation of detergent enzymes, the formulation of an enzyme is normally considered to be a way to store and transport the enzyme until its application. One common exception is enzyme immobilization, where the formulation is an active part of their application.
15.2.2 Glucose isomerase immobilization
Enzymes have been of great value to the starch industry since the 1960s, when the enzyme amyloglucosidase made it possible to completely break down starch into glucose. A few years later, most glucose production switched to the enzymatic process, which gave better yields, higher purity, and easier crystallization. Nowadays glucose isomerase is the most successful immobilized product used to convert glucose to high fructose corn syrup (HFCS) by isomerization of glucose to approximately 42 % fructose, which is fractionated chromatographically to 55 % fructose and 45 % glucose. The breakthrough for immobilized glucose isomerase for the production of HFCS was initiated by a number of factors. The intracellular glucose isomerase is expensive and cannot be applied economically without reuse. A solution to this is immobilization of the enzyme on an insoluble carrier that can be considered to be a particular form of formulation. It allows continuous processing in packed bed reactors, enabling control of substrate-to-product conversion by adjusting the flow rate. Moreover, particles with physical properties able to survive in large enzyme columns were needed to handle the huge volume generated by the starch industry, and no enzyme is left in the solution preventing contamination and toxicity problems. The enzyme particles are packed in large columns with packed bed heights of above 4 m and column diameters of up to 2 m. The substrate solution contains approximately 50 % w/w carbohydrates and is isomerized at around 60 °C to prevent infection of the syrup.
Numerous enzyme immobilization techniques have been described in the literature. The choice of a suitable immobilization method for a given enzyme and application is based on a number of considerations, including previous experience, new experiments, enzyme cost and productivity, process demands, chemical and physical stability of the support, and approval and safety issues regarding support and the chemicals used. The main immobilization principles are shown in Fig. 15.3. Enzyme characteristics that greatly influence the approach include size, surface properties, lysine content, and polarity. In immobilization by adsorption or via electrostatic interaction, the enzyme is adsorbed and interacts electrostatically with a large surface area of the carrier material such as alumina, clay, carbon, ion exchange resins, cellulose, or glass. In immobilization by covalent attachment, the functional groups of a number of amino acids are attached to the surface of a chemically modified support material. Those functional groups of the enzymes are used which can interact with other molecules such as amino and carboxyl groups. Synthetic polymers, cellulose, agarose, and porous glass are frequently used as support materials, and chemical agents are often used to link the enzyme to the support. In immobilization by occlusion or entrapment, the enzyme molecule is captured in a polymer matrix. Polyacrylamide, starch, gelatin, carrageenan, and alginate have been used for the polymer matrix. To stabilize the gels, crosslinking with bi— or polyfunctional agents may be necessary. Immobilization by direct crosslinking is an alternative. In immobilization by microencapsulation, the enzyme molecules are enclosed in polymeric capsules of 10—250 µm diameter.
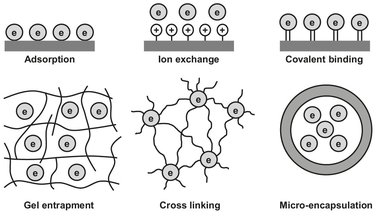
Fig. 15.3: Schematic representation of several techniques for immobilizing enzymes.
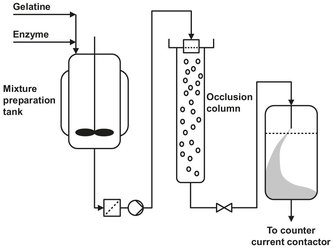
Fig. 15.4: Flow sheet of a production unit of immobilized enzymes.
The process, depicted in Fig. 15.4, uses a whole-cell immobilization technique. This is possible because the substrate to be converted (glucose) can easily diffuse into the destroyed cells. The immobilization principle is based on gel inclusion followed by crosslinking. The enzyme-containing cells are mixed with gelatin at a temperature of about 40 °C. The final gelatin concentration is about 8 % (w/w). The mixture is then prilled in a column containing a cold, water-immiscible organic solvent like butyl acetate. While dropping through the column, the enzyme-containing droplets will solidify. The spherical particles are collected at the bottom of the column, dehydrated, cross-linked with glutaraldehyde, and washed. Finally the product is sieved and preserved in propylene glycol. The same process can be used for immobilizing living cells. The used gel is permeable for substrate and formed products.
15.2.3 Detergent enzymes
At present the detergent industry is the largest user of industrial enzymes. The amount of enzymes in laundry detergent products is very high. Since the introduction of compact powder detergents, the use of enzymes as detergent ingredients has grown considerably. The trend towards even more compact products has changed enzymes from being minor additives to being key ingredients, in line with surfactants and bleach systems. Several changes in washing habits and detergent formulations underlie this development. Lower washing temperatures, required by modern textiles made from synthetic fibers and for reason of energy conservation, have caused a demand for more effective detergent ingredients.
Stains with good water solubility are easily removed during the washing process. All other stains are partially removed by the surfactant/builder system of a detergent, although the result is often unsatisfactory. In most case a suitable detergent enzyme may help. The catalytic nature of enzymes makes them highly efficient as detergent components. Contrary to the purely physical action of the surfactant system, enzymes work on degrading the dirt, which are often fats or proteins, into smaller and more soluble fragments. Typically only 0.5—2 % of granulated enzyme product is required in a compact powder detergent formulation. The actual enzyme content is in fact much lower, because enzyme granulates generally contain less than 10 % enzyme protein, the rest being inert encapsulation material. The resulting concentration of enzyme protein in the washing liquor will be in the range of at most a few milligrams per liter. Currently four different classes of enzymes are used in detergents:
· — Proteases are enzymes that are able to hydrolyze peptide bonds in proteins and therefore remove proteinaceous stains. Most detergent proteases are stable during the wash cycle in the presence of such active bleach systems.
· — Amylases catalyze the degradation of starch stains and improve cleaning by hydrolyzing the starch glue that binds other dirt and stains to fabric. They are fully compatible with detergent proteases and work together in the washing process. During storage in powder detergents, the amylases are very stable in the presence of proteases.
· — Lipases improve the removal of fats/oils of animal and vegetable origin.
· — Cellulases remove the cellulose fibrils from the surface of cotton fabric to improve the removal of particulate soil.
Not all enzymes with a potential for stain degradation and/or removal are suitable for use in detergent products. A detergent enzyme must have good activity at the pH of detergent solutions (between 7 and 11) and at the relevant wash temperatures (20—60 °C), and must be compatible with detergent components during storage as well as during the wash process. In particular, such an enzyme must be resistant toward protease degradation under these conditions. With enzymes like proteases and lipases a broad substrate specificity is demanded.
In the early days the enzyme was added to the detergent as a dry powder. However, various allergenic and skin reactions seemed to appear in people who worked in the production of the proteolytic enzyme. This problem was overcome by a proper formulation, which would encapsulate the proteolytic enzyme in order to prevent release of enzyme dust. Since then the washing process has changed considerably, in particular washing temperature. To an increasing extent the washing process occurs at lower temperatures. In this situation, however, the bleach components are less effective. This has led to the introduction of increasingly more bleachers and bleach activators (such as TAED, tetra-acetyl-ethyleendiamine) to facilitate bleaching at low temperature. As a result, the formulation had to offer protection against this type of aggressive component in the washing powder. At the same time, other market demands also arose with respect to the properties of the particles. These were required to dissolve quickly and be of uniform particle size with no release of odor and of a different color than the detergent used. Moreover, the formulation had to be adapted in such a way that the product would have a long shelf life in a relatively humid environment. Furthermore a form of controlled release was desired in order to prevent the release of the entire enzyme activity at once. Additionally, the different enzyme activities should not be released at the same time, because they are not compatible with each other. Therefore, multilayer formulations have been developed that bring the different enzymes in solution.
Today microorganisms grown in fermentors with volumes ranging up to several hundreds of cubic meters produce most industrial enzymes. After fermentation the enzyme is recovered from the fermentation broth. The first step is often the removal of whole cells and other particulate matter by centrifugation or filtration. Most microorganisms used in producing industrial enzymes release the enzymes from the cell into the growth medium, which facilitates the process. Ultrafiltration and diafiltration are increasingly used to remove water, salts, and other low molecular weight products.
After fermentation and recovery, the purified enzyme must be formulated into a dry product before it can be introduced into powder detergents. The challenge faced by enzyme producers is thus to provide a mechanically robust and rapidly soluble dry product. A common way is to precipitate the enzyme from the cell-free fermentation broth and collect the precipitated enzyme by filtration. The wet particles are then dried and sieved and the pure enzyme stored in powder form. As presented in Fig. 15.5, the first step in the enzyme formulation process is mixing the enzyme powder with several additives in a nonionic melt (ethoxylated-C18-fatty alcohol). The mixture is fed to a prill tower, where the droplets quickly solidify to give roughly spherical particles. The product obtained consists of matrix particles, i.e., the enzyme is uniformly distributed throughout the inert material. The coarse and fine fractions are remelted, while the proper fraction (250—600 µm) is coated to prevent dust formation during handling.
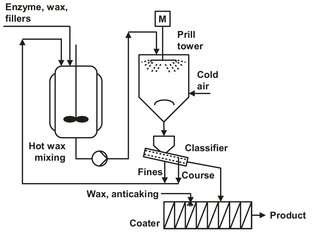
Fig. 15.5: Process for formulating dust-free detergent enzymes.
In layered granulates, inert carrier particles are used as a starting material. On the surface of these cores a layer of enzyme is applied, and on top of that additional layers can be applied, such as a coating layer to improve the dust properties. Today various kinds of fluidized beds are used in the production. On the basis of this fluid bed coating technology, Genencor International has developed a completely different technique. The starting point is a carrier material, such as sugar or salt crystals, which is sprayed with the enzyme solution in a fluid state while the solvent (water) is evaporated. When sufficient enzyme activity has been applied, a coating is sprayed. The advantage of this technique is that various layers with different enzymes can be applied. A disadvantage is that high demands are posed on the particle size of the carrier material and the correct dosing of the enzyme activity on the particles. A typical particle obtained from the fluid bed process is depicted in Fig. 15.6.
The different layers are clearly indicated. The nucleus consists of the sugar agglomerate. A layer of polyvinyl alcohol (PVA) is attached to strengthen the particle.
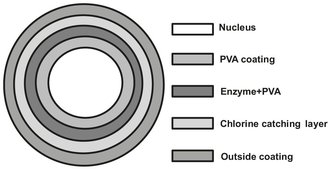
Fig. 15.6: Enzyme-containing granule with various coatings.
The enzyme is sprayed on this PVA layer. The enzyme solution also contains PVA to improve the adhesion on the particle. Subsequently an ammonium sulphate layer has been put up to catch the chlorine in the tap water during the washing process, because chlorine would directly deactivate the enzyme at the beginning of the washing process. The last layer consists of titanium dioxide or a pigment to give the particle its final color. Again PVA is added to this layer to minimize dust formation during handling.
15.2.4 Application research
In addition to formulation, application research is of utmost importance in the development and marketing of bioactive preparations. In application research it must be verified whether the product meets the requirements in particular cases of application and the formulation will have to be adjusted in light of the demands of particular customers.
After immobilization of glucose isomerase, many additional questions need to be answered before application of the immobilized enzyme. It is important to know the activity and lifetime of the immobilized enzyme as a function of the process conditions and its properties. This would include average particle size (distribution), pH, temperature, and substrate concentration. Because of its application in packed bed columns the product must have sufficient mechanical strength to withstand the force induced at high linear flow velocities. Furthermore, the immobilized enzyme must maintain its physical strength at temperatures of 60—65 °C. The rate of the equilibrium reaction between glucose and fructose can be described using Michaelis—Menten kinetics. Because the rate constant is temperature dependent, the reaction will go faster at higher temperatures. However, at the same time the deactivation rate of the enzyme will increase at higher temperatures. When both processes are combined the total productivity plot versus time in Fig. 15.7 is obtained. It can be seen that although the initial production rate is higher at higher temperatures, the total productivity is higher at lower temperatures because of less rapid enzyme deactivation.
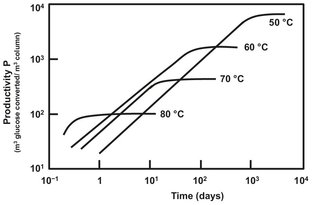
Fig. 15.7: Productivity of a commercial glucose isomerase as a function of operating time for various temperatures.
For detergents, it should be self-evident that the main part of application research on detergent enzymes is aimed at the contribution of the enzyme to the washing activity. Any laundering process is an interplay between the equipment used, the materials entering the washing process, and the procedure followed. Thus, elaborate washing experiments are necessary. A given enzyme may be assayed by its action on soluble substrates under chemical and physical conditions different from those encountered in a real-life wash. Such experiments indicate the enzyme’s performance with respect to pH and temperature variations, or in conjunction with other soluble substances, etc. Wash trials are carried out using solid test pieces. Laboratory wash trials are usually conducted in small-scale models of washing machines. The degree of stain removal is determined by the percentage of remission of light that is reflected from a dirty patch. Typically, the intensity of the light remitted at 460 nm when illuminating the test pieces with a standardized daylight source is expressed as a percentage, R, of the intensity of the incident light at the same wavelength. The Δ R-value is then a measure of the enzyme effect. It is defined as the difference in R between fabric washed with and without enzyme. The remission value is known to correlate well with the visual impression of fabric whiteness. Fig. 15.8 records the results of a protease performance test as a function of enzyme dosage. Detergent enzyme performance is often reported in the form of such a dose-response curve. The performance increases dramatically with enzyme concentration at the beginning, but reaches a maximum level at higher enzyme concentrations. Many washing tests are also carried out using ’real’ laundry to examine the effect of the enzyme.
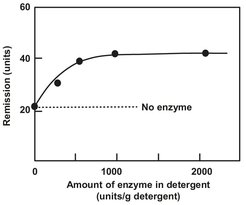
Fig. 15.8: Washing test for proteases.