Process Technology: An Introduction - Haan A.B. 2015
15 Product technology
15.3 Compounding
15.3.1 Introduction
Before a polymer can be used to make a product, it is usually mixed with various ingredients which serve a variety of purposes. The mixing processes also provide an opportunity to alter the physical form of the polymer, so that it is readily handled at the final conversion stage of its processing. There are two important reasons for compounding. The first is that additives are sometimes needed to alter the properties of the material, e.g. by making it harder or more flexible or cheaper. The second is that it is often important to prevent degradation of the polymer in service or during processing by means of appropriate additives.
Most of the polymers made by the larger polymer producers leave their factories as grains or powders which can be further processed by other manufacturers. From the original polymer, a variety of products with different properties can be designed by adding several additives. For example, dyes or ultraviolet stabilizers can be added. Because of the nature of polymers, special methods are needed to achieve a good dispersion of the additives in the polymer matrix. This chapter deals with several of these methods. For a better understanding of the processes, the thermal properties of the polymers need to be known. Most important is the temperature dependence of the modulus of elasticity, for which a typical dependence is given in Fig. 15.9. In this figure one can identify the glass temperature (Tg), melting temperature (Tm) or trajectory, and the rubber-plateau in between. The glass temperature represents the transition from a hard state to a rubber stage, where the polymer is more flexible. The polymer remains flexible over a range of temperatures represented by the rubber plateau. After further increasing temperature the polymer will melt, as can be seen from the figure. Polymers can only be processed in the rubber-state or when molten.
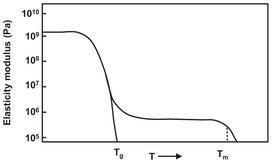
Fig. 15.9: Modulus of elasticity of a polymer as a function of temperature.
15.3.2 Compound formulation
Since desired properties for materials vary over a broad range, it is likely that a pure polymer does not meet these demands. The polymer may not be strong enough, have the wrong color, or might be too expensive to be used as a pure substance. Fortunately, the properties of polymers can be adjusted to meet almost every need by adding other polymers, plasticizers, dyes, fillers, antioxidants, and so on. These additives must be incorporated into the polymer before final shaping takes place; this step in the production is called compounding. The typical equipment used in compounding will be discussed in the next section. This section deals with the formulation of the mixture supplied to the compounding-process.
Probably the best-known example of formulation is the addition of soot to rubber. This is done to reduce the wear of the polymer. Without the use of soot as a filler a car tire has to be replaced after 10 000 km, while with the addition of soot this can be up to ten times as long. This is mainly due to the very high surface area of the soot, which efficiently levels out the tension on the polymer. In general the addition of particles, like soot, to a resin improves the stiffness and not the tensile strength, as is the case with soot in rubber. The price of the raw material can also be a reason to formulate a cheaper mixture of polymers that approaches the properties of the (more expensive) single polymer. It is also possible to improve the properties of a cheap resin by adding a polymer with better properties. If the polymers are miscible on a molecular scale, the properties of the mixture can be gradually varied between the properties of both polymers. This way a broad range of materials can be designed. Molecular miscibility of polymers is more an exception than a rule; dispersions are more common. Dispersions consist of a continuous phase with a second phase (finely) divided in the first phase. Ideally the second phase consists of fine droplets in the continuous phase, but often the droplets are not completely spherical. Properties of dispersions also vary between the properties of the pure polymers, but the relation is not as straightforward as is the case with miscible polymers. For example, the modulus of elasticity exhibits different relations for real mixtures and dispersions (Figs. 15.10a and 15.10b). As can be seen from Figs. 15.10c and d, it is also possible to achieve different relations if the polymers are partially miscible.
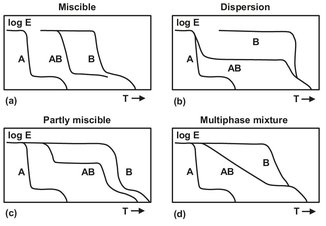
Fig. 15.10: Modulus of elasticity for various polymer mixtures.
Adding fibers to the polymer, which also increases the tensile strength in most cases, can also influence the modulus of elasticity. The fibers added can be either short or long, and oriented or disoriented. The fiber length should be long enough to be able to take the pressure from the polymer matrix. Disoriented fibers result in a toughening of the polymer in all directions. When the fibers are oriented in one direction, this is the only direction in which reasonable toughening takes place.
15.3.3 Additives
Different additives can be used to improve the properties of polymers. Three main groups can be distinguished. Additives that
· (1) protect plastics against degradation and aging like antioxidants, UV stabilizers, and heat stabilizers;
· (2) facilitate or control processing such as lubricants, mold release— and blowing agents;
· (3) impart desirable new qualities to plastics like pigments, dyes, and flame retardants.
In addition to the chemical and physical properties, economics also play a large role in selecting the optimal additive for a specific polymer. The most important additives are discussed in this section.
Protective additives
Although a very large number of additives from this category are used, the most important groups are antioxidants, heat stabilizers, and UV stabilizers. Antioxidants are used to protect the polymer structure against atmospheric oxidation and minimize associated damage (e.g., discoloration, reduction in gloss, cracking, embrittlement) during processing and the service life of the final product. Many polymers have sites on their molecular chains that are susceptible to attack by oxygen, often catalyzed by catalyst residues and contaminants. Exposing the polymer to high temperatures and severe shear during production and processing accelerates the oxidation reactions. It is the function of antioxidant additives to combat oxidative attack, by interfering chemically with the series of reactions leading to chain scission. Most antioxidants are themselves oxidized and consumed when performing their function, so that the oxidation behavior of the additive in a given polymer is crucial for its effectiveness. Important antioxidants include sterically hindered phenols, secondary aromatic amines, sterically hindered amines, phosphites, and metal salts.
Heat stabilizers perform a similar function in preventing degradation at high processing temperatures. Their function is to stop other types of degradation reactions, for example the tendency of some polymers to depolymerize. Combinations of stabilizers and costabilizers are normally used to obtain optimal results. These additives are particularly important in the processing of PVC, which readily degrades and darkens when heated under the creation of hydrogen chloride. Heat stabilizers for PVC are divided into metal-containing and metal-free costabilizers such as phosphates, epoxy compounds, and polyols. Typical stabilizers include heavy-metal compounds containing lead, organotin, cadmium, or zinc. The choice of a stabilizer system depends on the applied processing methods, the intended use, and the compatibility with other additives.
UV stabilizers are mainly employed to extend the service life of plastics for outdoor use, such as garden furniture, stadium seating, window frames, floor coverings, films for protective coverage and agriculture, light fixtures and illuminated signs, and automotive parts such as bumpers and body paints. UV stabilizers often work in conjunction with antioxidants, because the attack at the reactive site on the polymer by UV radiation in sunlight is one of the reactions which initiate oxidative degradation. Many plastics therefore suffer from yellowing, surface cracking, embrittlement, reduction of shininess, or chalking after a short time outdoors and ultimately disintegrate. UV stabilizers can be incorporated in the bulk plastic or applied as a coating. They work by absorbing UV preferentially and reemitting the energy harmlessly at a lower wavelength. Ideally, a UV stabilizer should not be consumed, but should operate in a closed cycle, so that it remains in its active form even after a long period of weathering or use. Typical concentrations used range from 0.05 to 0.5 %, reaching 3 % in some critical applications.
Processing aids
Plastic surfaces can become electrostatically charged by friction during processing or use. Such charges can make processing difficult, and their discharge can be unpleasant. Discharges can in some cases even create a potentially dangerous spark. Surface-active antistatic agents are used to prevent the buildup of undesirable static charges by creating a conductive surface layer that allows the charge to dissipate quickly and prevent it from building up. In some cases, other additives must be used to impart volume conductivity throughout the bulk of the plastic. Surface-active antistatic agents are applied by solution spraying or incorporated into the plastic mass. They are typically used at levels of 0.1—2.5 %.
Processing lubricants and mold-release agents are widely used to assist the passage of material through the processing machinery. They are often oils and waxes, classified into internal and external lubricants. Internal lubricants lubricate the polymer granules during processing and allow easier and cooler melting. These materials are often at least partially miscible with the polymer melt. External lubricants are essentially immiscible. They lubricate the mix against friction with the processing machinery, allowing the correct degree of friction for the process to work while preventing too much friction that will cause local high temperatures and degradation. Lubricants also improve the dispersion of pigments and fillers in plastics resulting in more uniform colors and improved material properties.
Property modifiers
Property modifiers, as their name suggests, alter the physical properties of the polymer. There are many types, and several examples include reinforcing and nonreinforcing fillers, chemical additives, other polymers, flame retardants, and colorants. Nearly all thermosetting and thermoplastic resins are filled or reinforced for special applications. Filled (or reinforced) plastics contain large amounts (20—50 %) of fillers, divided into extenders, which are added to cheapen the mix, and reinforcements, which improve several of the polymer properties. Calcium carbonate and china clay are quantitatively the most important extenders. Other fillers include ground dolomite, calcium sulfate (gypsum), barium sulfate, wood flour, cork flour, starch, metal oxides, and metal powders. Coupling agents improve the adhesion between filler and polymer, preferably via chemical bonds. Their use confers reinforcing properties on inexpensive extenders, improves the performance of reinforcements, and allows the filler content to be increased. Important examples of reinforcing fillers are carbon black added to rubbers and fine particle silica. Carbon black is obtained by incomplete combustion of gaseous and liquid hydrocarbons. Silica is used as a natural crystalline product (sand, quartz powder) or amorphous synthetic product. The reinforcing effect of fillers depends on their chemistries, shapes (fibers, flakes, spheres) and sizes (fiber length, particle size). A wide variety of inorganic and organic fibrous fillers and reinforcements are used. Important examples are short (3—6 mm) carbon and glass fibers. Glass fibers are milled to short fibers (e.g., 0.2—0.6 mm) when the reinforcement is blended with the polymer in the extruder.
A second important example of property modification is the addition of small amounts of another polymer as impact modifier. Because several plastics, such as PVC, polyolefins, or polystyrene are brittle, impact modifiers are used to improve impact strength, especially at low temperatures. Impact modifiers are elastomeric copolymers with low glass-transition temperatures. They are dispersed as discrete soft phases in the thermoplastic polymer. Transfer of impact energy to the elastomer phases requires not only a good distribution but also adhesion at the interface between the two phases by chemical bonding or physical cross-linking. Important examples are methyl methacrylate-butadiene-styrene copolymers, graft copolymers of acrylonitrile and styrene on polybutadiene (ABS), ethylene-propylene-diene copolymers (EPDM), and styrene-butadiene rubber (SBR).
The application of combustible plastics in buildings, equipment and vehicles requires the incorporation of flame-retardants to reduce the fire risk. In plastics, flame-retardants reduce ignitability, combustion rate, and heat release. Although flame-retardants can be especially effective in the early stages of a fire and can reduce flammability, plastics modified with these additives are still combustible. Because flame-retardants generally lower the quality and increase the price of a plastic, they are only used when mandated. Selection criteria are the effectiveness and price of the flame retardant, processability of the plastic, and influences on the polymer properties. Flame-retardants may be inorganic substances, halogenated organic compounds, organophosphorus compounds, or other organic substances.
Colorants (dye, pigment) are added for the decorative character of the polymer. They are chosen from a wide range of organic and inorganic compounds. Pigments are particulate organic and inorganic solids that are virtually insoluble in the polymer and must therefore be dispersed in the matrix. Many color formulations are derived from a combination of inorganic and organic pigments whose properties complement one another. Dyes are organic compounds that usually dissolve in the substrate. Polymersoluble dyes are usually suitable for transparent plastics, but not for polyolefins. Incompatibility is manifested by “crocking”, the migration of colorant to the free surface of the plastic, from which it can be rubbed off. Colorants are introduced into plastics by several methods. The dry colorant may be blended with the polymer before processing (dry blending) or through the addition of concentrated predispersed colorant pellets (masterbatches). Masterbatches offer numerous advantages over powdered colorants, particularly improved dispersion and dissolution of the colorants, and more hygienic working conditions.
15.3.4 Polymer-mixing mechanisms
Most of the polymers produced undergo processing that involves mixing in one way or another, for example for the addition of stabilizers, or when another resin is added to achieve the desired properties. The best (or most constant) properties of a dispersion are usually achieved when the dispersion is as homogeneous as possible. As mixing is such an important feature in polymer processing, a general idea of the mixing process is essential. In general the purpose of mixing of polymers will be to achieve a sufficiently stable dispersion of two nonmixable resins. As is always the case with nonmixable liquids, the dispersion will consist of a continuous phase and a phase dispersed as droplets in the continuous matrix. Mixing in this is case is essentially the deformation and breaking-up of the droplets in the original dispersion. The two main driving forces are shear stress on the dispersed phase and interfacial tension of the dispersed phase with the continuous phase. Depending on the ratio of these two forces, two different mixing processes can be observed: distributive and dispersive mixing. The first process mainly involves deformation of the droplets, while the second mainly involves size reduction of the dispersed phase. The size of the droplets in, for example, an extruder is reduced from several millimeters at the feed to the micrometer range at the die.
Distributive mixing
If the shear stress on the droplets of the disperse phase is very large compared to the surface tension of the droplets, deformation of the droplets takes place (almost) exclusively. During this stage, the droplets are deformed due to the shear stress applied. Since the shear stress in an extruder varies with the distance from the wall, the droplets are deformed to an ellipsoid. Ultimately, the droplets will be deformed into a long strand of polymer (which is actually a very long ellipsoid), which of course has the same total volume as the original droplet. If the “droplet” is perpendicular to the direction of deformation, the effect of deformation will be as large as possible. During deformation, the shape of the droplet will change to a strand, which is oriented in the direction of the shear-stress. Therefore in most applications the droplets (and matrix) are folded and reoriented after the first stretching step. Apart from improving the effectiveness of the shear stress applied, it also improves the speed of deformation, as can be seen from Fig. 15.11. Instead of a linear increase in length, the increase becomes exponential, speeding up the process.
In practical situations, the equipment used is designed to perform this stretching and folding in one way or another. The Sulzer SMX is an often used mixer, because of its good mixing properties and low pressure drop.
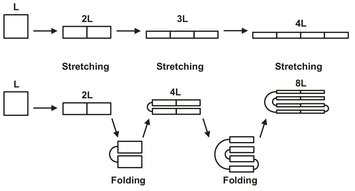
Fig. 15.11: Deformation with and without folding.
Dispersive mixing
The diameter of the strands formed during distributive mixing will decrease with time as long as the shear stress is applied. In this process the time and level of shear stress are interchangeable. A long time with a low shear stress will have the same affect as a high shear stress applied for a short time. Ultimately the strand will reach a diameter at which it will break up into several droplets. The formation of these droplets is not yet completely understood, but several mechanisms have ben empirically found. Of course, the diameter of the strand will not be completely homogeneous: some parts will be larger, some smaller. It has been shown that if the wavelength at which these inhomogeneities appear is larger that the circumference of the original strand, the amplitude will grow. If the wavelength is smaller, the amplitude will eventually approach zero. If the amplitude reaches the value of the original diameter of the strand, the strand will break, and droplets are formed. In this description, the strand is assumed to be at rest and there is no flow around it. If there is a flow around or along the strand, the process remains almost the same. The only difference is that the growth of the amplitude will be suppressed by the flow. Because of this, more time is needed before the strand breaks up, and the resulting droplets will be smaller, since the strand has been stretched more.
15.3.5 Compounding equipment
Since almost all products require additives in the polymer, it can be understood that compounding is a vital step in polymer processing. The main goal of compounding is mixing all the ingredients necessary to achieve the desired properties and to result in mixture as homogeneous as needed for the final application. Frequently used methods for compounding are roll-mill mixers, banbury-mixers and extruders.
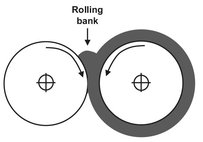
Fig. 15.12: Two-roll mixer.
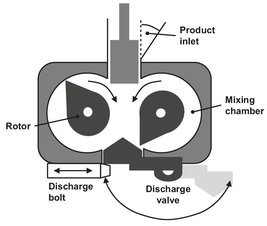
Fig. 15.13: Banbury mixer.
The simplest machine for intensive mixing is the two-roll mill (Fig. 15.12). Roll-mill mixers consist of two steam— or oil-heated counter-rotating rolls, with a small gap between them. It mixes the polymer at a relatively high viscosity corresponding with the rubber plateau in Fig. 15.9. The two rolls have different rotation speeds and the polymer bypasses the faster roll and again enters the gap between the rolls. When a two-roll mill is used for mixing, the objective is to pass the appropriate loading of matrix material through the nip a few times until it warms up, softens, and forms a smooth band round one of the rolls. The nip is adjusted once the band is formed to give a small ’bank’ of polymer rolling along the top to the nip. As soon as this condition is achieved, the additives are introduced, and the mill starts to incorporate them into the material. This mixing process is assisted manually by cutting the band with a knife, so that a flap is formed which can be folded to the other side. To achieve a relatively homogeneous mixture, cutting and folding has to take place up to 20 times. The resultant mix is an intimate one, below the resolution of the eye. Two-roll mills are good at dispersive mixing in the radial direction but poor at distributive mixing along the length of the rolls. The Banbury mixer is the best-known mixer. Nowadays it is mainly used in the production of polyolefins, whereas it was originally designed for rubber compounding. It is very useful when highly viscous polymers have to be blended. This type has two rotors turning in the opposite direction which fit very tightly to the vessel walls. A compression ram is used to confine the polymer inside the mixing compartment (see Fig. 15.13). Because of the very high friction, temperature can rise very quickly in the mixer, so that cooling is needed. After a predefined period of time, the lumps of polymer are discharged, and another batch of polymer can be mixed.
Since polymers have to be melted before they can be extruded, extruders are often used to combine, compounding with the actual extrusion process. The most widely used machine is the single-screw extruder shown in Fig. 15.14. The single-screw extruder is primarily a drag pump, suitable for working with highly viscous fluids and capable of operation at high pressures and temperatures. The design is mechanically simple and consists essentially of a screw fitting closely in a cylindrical barrel. The screw and barrel can be modified to meet the specifications needed for specific polymers with content of different viscosity and volatility. The usual screw configuration has 20 or more turns with a pitch similar to the diameter, giving a long slender machine. Solid polymer is fed in at one end, and the molten extrudate emerges from the other. Inside, the polymer melts and homogenizes. In the feed zone the screw depth is constant and the solid polymer is preheated while conveyed at a steady rate to the subsequent zones. Since it is a mixture of solids (polymer) and gas (usually air), the feed has a low bulk density and a relatively high volume. This is the reason for the high channel volume in the first section. The second zone has decreasing channel depth. This compression zone melts the solid polymer particles by the shearing action of the screw, expels the air trapped between the granules, improves heat transfer from the heated barrel wall, and accommodates the density change during melting. A consequence of decreasing channel depth is an increase in pressure along the extruder (Fig. 15.9), which finally forces the molten polymer through the die. In the metering zone we again find a constant screw depth. Its function is to homogenize the melt and supply it to the die zone. The drag flow, or volumetric conveying capability of the extruder for the plastic melt, depends only on the screw speed, N, and the geometry, A, of the screw:
![]()
(15.1)
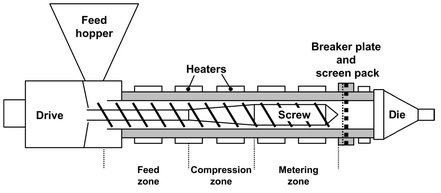
Fig. 15.14: Mean features of a single-screw extruder.
At the discharge end of the metering section, sufficient pressure must be generated in order to overcome the resistance of the transfer piping and the shaping die. This pressure causes the plastic melt to try to flow back down the channel and possibly also over the flight tips. This pressure flow is dependent on the screw geometry, B, pressure driving force Δ P, filled length of the screw, L, and melt viscosity, η:
![]()
(15.2)
The net flow of an extruder is simply the difference between drag and pressure flow:
![]()
(15.3)
Several additional screw sections can be incorporated into the metering zone, such as a stage for venting volatiles, or pins to improve the mixing action. In addition to distributive mixing, high shear stresses over the flight tip give a degree of dispersive mixing for breaking up solid agglomerates such as pigments. The drag mechanism also causes internal shear of the viscous material being pumped, leading to temperature rise in the polymer. This inefficiency is utilized beneficially in melting the polymer by internal rather than external heating. The single-screw extruder is highly suitable for continuously processing a wide range of synthetic thermoplastic polymers into finished or semi-finished products. Due to the reasonably good mixing in the extruder, they are even used only for compounding if the final product has to be produced by another method. This is mainly due to ease of operation and low capital costs.
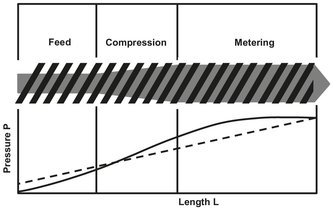
Fig. 15.15: Zones in a single-screw extruder.
Several companies produce extruders in which adjustments have been made to improve the mixing abilities compared to a “normal” extruder. Two designs frequently applied in compounding processes are the Buss-Ko kneader and the twin-screw extruder. As illustrated in Fig. 15.16, the Buss-Ko kneader is an extruder with stationary pins attached to the barrel and interrupted screw flights. This way high shear is generated in the narrow gap between pins and screw flights. A recirculating flow is generated which provides very good dispersive mixing and thus an excellent control over the melt temperature. The Buss-Ko kneader is used for a wide range of polymers, but is particularly successful with temperature sensitive materials such as PVC and thermosetting plastics.
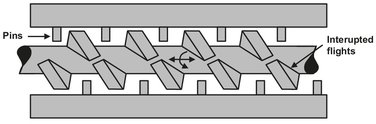
Fig. 15.16: Buss-Ko kneader.
Twin-screw extruders are used where superior mixing or conveying is important. Instead of a cylindrical barrel, the twin-screw extruder has an eight-shaped barrel to accommodate the two screws. It is characterized by its two main properties: the rotation of the two screws and the position of the screws. The screws can be either corotating or counterrotating, and they can be positioned either intermeshing or nonintermeshing. As their names indicate, the difference is in whether the two screws rotate in the same direction or in opposite directions. The nonintermeshing types consist of essentially two single screws side by side and work in a similar manner to single-screw machines. The majority of twin-screw extruders have the two screws intermeshing to a greater or lesser extent (Fig. 15.17).
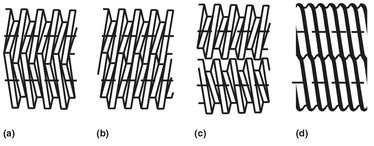
Fig. 15.17: Types of twin-screw extruders: (a) intermeshing counter-rotating; (b) intermeshing corotating; (c) nonintermeshing counterrotating; (d) sinusoidal self-wipingscrews.
The twin-screw extruder acts as a positive displacement pump whose output is roughly proportional to speed but less affected by back pressure and friction. A general advantage of using twin-screw extruders is the fact that they have a larger heat transfer area available and thereby provide better temperature control. For compounding, the corotating extruder with low conjugation is the best option, since it has the best mixing properties. The maximum rotation speed of the screws can be used as an indication of the mixing abilities of the extruder. If the rotation speed is high (about 300 rpm), the positive conveying in the extruder is poor, and this is generally an indication of better mixing properties. If a self-wiping extruder is used (Fig. 15.17d) with a relatively high degree of openness, these effects are larger, and the rotation speed can be up to 600 rpm. The twin-screw extruder is thus good for compounding, especially for heator shear-sensitive polymers. However, its high initial cost and relatively low output are obstacles to its use where the single-screw machine can approach its performance.