Process Technology: An Introduction - Haan A.B. 2015
Appendices
Polypropylene
B.2.1 Gas-phase process
The process most used in gas phase polymerization of propylene is the Amoco/Chisso process, utilizing a horizontally stirred bed (Fig. B.6). It can be used for a variety of polymerizations, but is particularly suitable for the polymerization of ethylene and propylene. In this process, the monomer is added to several separate sections of the polymerization reactor, optionally together with a certain amount of hydrogen. The temperature in the sections may vary from each other and is usually between 40 °C and the softening point of the content of the reactor. Each section contains a certain amount of polymer particles with a certain degree of polymerization and particle size. Polymer product passes through all the beds to obtain a product with the desired properties. The particles in every section are severely agitated using impellers with two paddles each, the paddles of two adjoining impellers placed in a 90° position relative to the other. The paddles are designed to stir the bed while not moving the particles to the adjacent bed. Stirring takes place to achieve mass and heat transfer at a sufficient level. The polymer product is transferred to the next bed through a take-off barrier opening, as shown in Fig. B.7. The reactor is operated isobarically and continuous cooling and product removal takes place. All the beds are continuously quenched for cooling purposes; the liquid used for quenching is usually isobutane or isopentane. Often the catalyst is supplied together with the quench, either to all beds or to a smaller number of beds. The catalyst consists of two compounds, one being a trialkylaluminium chloride, the other an activated titanium trichloride. The amount of catalyst added can be used to control the particle size of the polymer.
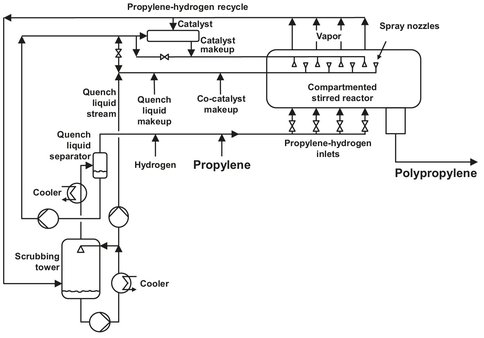
Fig. B.6: Flowsheet of the Amoco/Chisso polymerization of propylene. Adapted from [212].
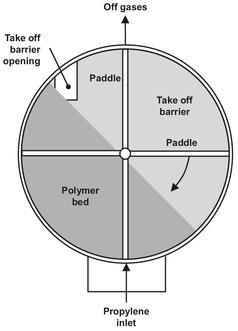
Fig. B.7: Cross section of the horizontal stirred bed polymerization reactor.
The off-gasses are purified before recycling to the reactor again. This step includes the removal of polymer fines and catalyst components. After their removal, the off-gasses are led to a scrubber to remove the quench-liquid, which is partially recycled after cooling. The major part of the liquid is used to scrub the incoming off-gasses. The overhead of the scrubber is combined with the fresh monomer feed and enters the reactor below the polymer particle bed.
The produced polymer is transferred to the take-off vessel, almost without a pressure drop. In this vessel, the polymer is reacted with an extra amount of monomer. This operation takes place adiabatically, and the heat produced is used for melting the polymer. Together with external heat this results in a molten polymer, which is easier to handle than in the solid state. The polymer is devolatilized and converted to the appropriate commercial size product. Water is added to the molten polymer, thereby killing the catalyst to prevent unwanted reactions after the polymer has left the plant. This is also the point were several additives and coloring agents are added to the polymer.
B.2.2 Slurry process
The process described, shown in Fig. B.8, was originally designed by Montedison S.p.A. and Mitsui Petrochemical Industries, Ltd. Due to the improved activity of the catalyst, it was possible to simplify the original slurry process, which involved polymer washing to remove catalyst particles and alcohol. The process consists of four steps: polymerization, preceded by catalyst preparation and monomer and catalyst metering, phase separation and drying, compounding and pelletizing including the addition of stabilizers and solvent recovery.
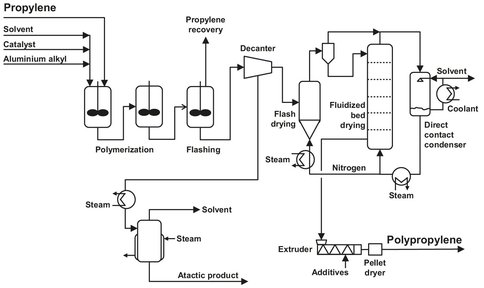
Fig. B.8: Polypropylene slurry process. Adapted from [211].
Catalyst preparation
In the polymerization reaction, which is carried out in a solution in heptane, a catalyst is needed. The catalyst used in this process is a two-component catalyst, one consisting of a tri-alkyl-aluminium compound and the other mainly containing activated magnesium or manganese chloride. This system has proven to be a highly active and stereo-specific catalyst, and yields can be as high as 1 million grams of polypropylene (PP) per gram of catalyst. Due to this high activity, there is no need for a catalyst removal step, adding to the simplicity of the process.
The catalyst is prepared just before it is used to ensure high activity and fresh catalyst. The aluminum-containing compound of the catalyst can be made by various methods, all beginning with a tri-alkyl-aluminium compound. Addition of a Lewis base, such as pyridine, diethylether, and tetrahydrofuran, yields the desired product. The magnesium— or manganese-containing compound can also be made by several methods, one of which is to combine it an alkyl-aluminum compound. The magnesium chloride, which is used most often as a support, is dry milled to achieve a surface area greater than 3m2/g and preferably greater than 20 m3. During the dry milling a titanium-containing substance, such as TiCl4C6H5COOC2H5, is added. The Al/Ti-ratio is between 10 and 1000, depending on the desired product specification. To ensure a good stereoregulation, a weak stereoregulator is added to the tri-alkylaluminum compound. An example of this stereoregulator is dimethylether, which is used most often. In addition to this weak regulator, a strong stereoregulator is added directly to the reactor. The most used strong regulator is p-ethoxybenzoate.
Polymerization
Polymerization takes place in a cascade of two identical reactors which are operated at 75°C and 16 bar. The reactors have a length-to-diameter ratio of 2: 1 and are equipped with high intensity agitators and internal cooling coils. The walls of the reactor are also cooled to ensure a high enough heat transfer, thus avoiding the melting of the polymer particles formed. During operation, the reactors are completely filled with liquid. The solids concentration in the outlet of the first reactor is about 32.5 wt% and is used as the inlet for the second reactor. Because of the lower monomer concentration in the second reaction vessel, the conversion is lower. The overall conversion is about 97 %, of which 83 % is achieved in the first reactor.
Phase separation and drying
First the unreacted propylene is recovered by flashing and storage for further use. This is done in a flash vessel, which operates at 1 bar, followed by a small distillation unit. The propylene stream also contains some propane, which can be present in the propylene feed. The remaining slurry is centrifuged to remove the bulk of the diluent. After this step the volatiles content of the polymer is about 30 wt%. It is transferred to the primary flash dryer, which operates under a closed nitrogen circulation at 1 bar and 116 °C. The volatiles content of the polymer is lowered to about 5 wt%. The temperature of the inlet gasses is limited to 130 °C to prevent polymer melting. The partially dry polymer is transferred to a final drying stage. This is done in a fluidized bed, where virtually all hydrocarbon diluent is removed from the polymer. The fluidized bed also operates under a continuous nitrogen flow, from which the evaporated diluent is removed in a scrubber. To achieve the low volatiles content in the polymer, the scrubber operates at low temperatures (—18 °C) to remove all diluent from the nitrogen recycle.
Compounding and pelletizing
This step is conducted in a conventional way, as discussed earlier. The crude polymer is added to an extruder, and molten and additives are introduced if desired. In this section, homogenization takes place as well.
Solvent recovery
The solvent from the several drying stages has to be purified before recycling. This is done in a simple two-column distillation unit to remove heavy ends and monomer and diluent recovery. After drying, the diluent can be recycled to the diluent vessel.
B.3 EPDM
Because of the limited production of natural rubber, or the difficulties in obtaining it, the search for synthetic rubber began. Several types of synthetic rubber have been developed, among which are EPDM (ethylene-propylene-diene-monomer). In EPDM production a third monomer is usually added to introduce unsaturated bonds, which can be used for vulcanization (crosslinking) of the rubber. The third monomer often used is ENB (5-ethylidene-bicylo(2,2,1)heptene-2), which is the unconjugated diene shown in Scheme B.1. Important used of EPDM include tires, footwear, and plastic modification.
EPDM is produced in a continuous process, of which the solution process in the most commonly used. A process also used by a number of producers is the slurry process, which has lower catalyst and steam cost, but provides a lower flexibility in product specification and a lower homogeneity of the EPDM due to diffusion limitations. The details of the process vary a lot for each manufacturer and are highly proprietary, as can be seen from the number of patents related to this production process. The catalyst used can be divided into two parts. The first is a transition metal halide, such as TiCl4, VCl4, and VOCl3, which is used most often. The second part is a metal alkyl component such as (C2 H5)AlCl2 or (C2H5)2AlCl. Both components are very sensitive to water, and therefore only a few ppm of water is allowed in the feed streams. This catalyst system is completely soluble in hexane and therefore has a higher activity than heterogeneous catalyst systems. This can be explained by the lack of polymer-film formation on the catalyst surface: there is no real catalyst surface at all. The product of the reactivity ratios for ethylene and propylene is almost unity, indicating that the polymer formed will be almost perfectly random.
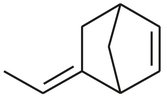
Scheme B.1:5-ethylidene-bicyclo[2.2.1]heptene-2 (ENB).
A flow sheet of a typical EPDM production process is given in Fig. B.9. Most manufacturers use a solution in hexane. In this solution process ethylene and propylene are mixed; a common composition uses 40 wt% of the latter. Before mixing the monomers are purified, dried, and cooled to —30 °C. The gas mixture is added to an organic solvent, to a concentration varying from 3 to 8 wt%. Optionally the third monomer is added, and the solution is led to the reactor. If ENB is used, it is first purified to remove the polymerization inhibitors. This is done by washing with a caustic solution, washing with water, and drying. The reaction takes place in two well-stirred vessels at about 60 °C and 25 bar in the presence of a catalyst system. The vessels operate in series, and optionally fresh monomers and/or catalyst is added to the second vessel. The polymerization itself is exothermic and very fast, with the lifetime of a single growing chain being a few seconds at most. The heat produced has to be removed to ensure the right reaction conditions for obtaining a product with the desired molecular weight and distribution. This is done by applying a cooling jacket filled with cooled water.
The exit stream of the second reactor is mixed with polypropylene glycol to terminate the reaction. In a flash vessel almost all of the unreacted ethylene is removed and recycled to the reactor together with recycled hexane. The rubber solution contains about 10 % of EPDM and some unreacted olefins, all in homogenous solution. The solution is mixed with superheated water to remove the catalyst from the EPDM. The aqueous layer that settles out is partly discarded (10 %), and the remainder is recycled. The dispersion is then steam stripped to remove all of the solvent, and the rubber crumbs are formed. To obtain a dry product, the slurry of rubber crumbs is pumped over a shaker screen to remove excess water. The crumb, which now has a water content of 50 wt%, is then fed to the first stage of a mechanical-screw de-watering and drying press. Here the water content is lowered to 3-6 % as the rubber is pushed through a perforated plate. After another drying stage, where the temperature of the rubber reaches 150 °C, the remaining water is flashed off. A final step in the drying process takes place in a fluidized bed at a temperature of 110 °C. After this step the remaining volatile matter is lower than 0.3 wt%. The EPDM crumb is then weighed, pressed into bales, and packed for storage and shipment.
The vapors from the flash vessel are partly condensed and separated into two layers. Both layers are sent to a distillation section for recovery of monomers and solvent. The organic layer is distilled, and the resulting solvent-rich and ENB-rich streams are sent to various other distillation columns. The solvent is first freed of water, and after that 1-hexene is removed. After drying over molecular sieves, the solvent is recycled. The heptenes and higher olefins are removed from the ENB by distillation and before recycling. The ENB dimer is removed by vacuum distillation.
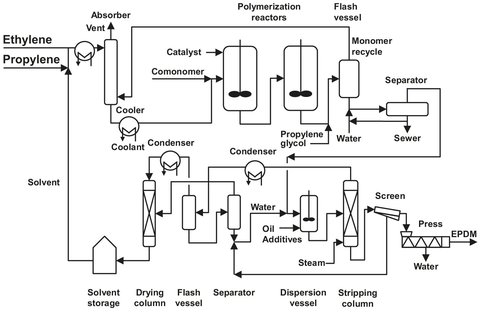
Fig. B.9: Typical EPDM process flow sheet.
B.4 Polyamides
B.4.1 Introduction
Polyamides (nylons) can be produced in a wide range of compositions, properties, and prices. Because of this they are a group of polymers that are commonly used. Nylons can be divided into to types: homopolymers (like Nylon 6) made from one monomer, and heteropolymers (like Nylon 4,6) made of two different monomers. These two types are illustrated in Scheme B.2. The numbers added to “nylon” indicate the number of carbon atoms in the monomers used and thus to the type of nylon. Nylon 4,6 has a very high melting point (290 °C) and a high strength compared to other polymers. Therefore it is used as a heat resistant material and for the production of cogs applied in a high temperature environment.
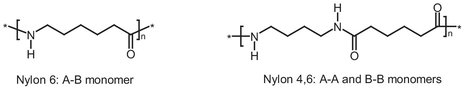
Scheme B.2: Different types of nylons.
B.4.2 Nylon 6
The production of Nylon 6 is one of the few large-scale industrial exploitations of a ring-opening polymerization. The monomer used is caprolactam, the production of which has been discussed in Appendix A. For the initiation of the polymerization, the presence of water is essential, but for the completion of the reaction water has to be removed in order to obtain a maximum yield of Nylon 6. Almost all major Nylon 6 producers use a continuous process know as the VK-process, which is shown in Fig. B.10. Most of the producers have modified the process to their liking, thus producing the desired quality, composition and properties. The process was originally developed in Germany by BASF, and its full name is the vereinfacht kontinuierlich (=simplified continuous) tube process.
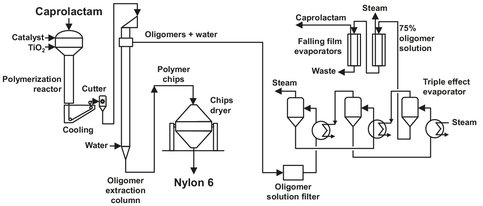
Fig. B.10: Nylon-6 VK-type process flow sheet. Adapted from [213].
In this process caprolactam, titanium dioxide (TiO2) and steam enter the VK tube at the top. The water content can be raised up to 15 wt% in modern processes to ensure an acceptable polymerization speed. Today, prepolymerization is carried out in two VK tubes in series, the first operating at a pressure of more than 1.5 bar, the last operating at atmospheric pressure. This is done to shorten the reaction time relative to the one VK tube process. The upper part of the reactor has a temperature of 240—280 °C at atmospheric pressure. Usually the upper part of the reactor has a 40-60 % larger diameter, and the reactants are introduced near the outer walls of the tube. After a quarter of the reactor (the part with the larger diameter), almost all of the water has been removed from the mixture and carried off overhead. The water is purified and returned to the reactor. The resulting prepolymer, usually containing 10—14 monomer molecules, is fed to the lower part of the reactor. This section also operates at temperatures of 240-280 °C, resulting in a product leaving the reactor in a molten state. Residence time in this section of the reactor can be several hours. After passing a filter to remove unwanted particles, the product is cast and granulated after cooling in water. Before solid state postpolymerization, the pellets have to be carefully dried in order to obtain high molecular weights in the postpolymerization. This is done using oxygen-free nitrogen to remove any water vapor. Solid phase condensation takes place at 130-180 °C, either in vacuum or in an inert gas stream.
B.4.3 Nylon 4,6
In the 1980s, DSM introduced a new commercial aliphatic polyamide, Nylon 4,6. It was first studied in the 1930s, but no further research was done because of the difficulties in obtaining high molecular weights and degradation of the product. The two-step process developed by DSM (Fig. B.11), however, settles these problems. Raw materials used are adipic acid and diaminobutane, or the salt of those materials. First, the salt of diaminobutane and adipic acid has to be produced. This is done by mixing molten diaminobutane with the salt solution. To this solution, a 47% solution of adipic acid in water is added. The major part of the solution formed is passed through a heat exchanger and added to a reaction vessel at a temperature of 98 °C. The minor part is diluted with water for pH monitoring, which is the major control parameter for diaminobutane addition. The salt formed in the reactor is removed at the rate it is formed by an overflow in the reactor.
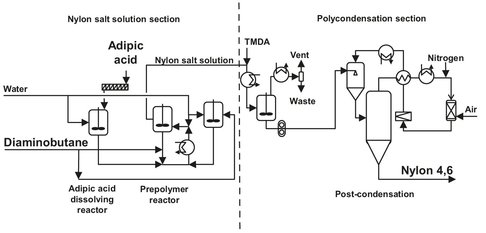
Fig. B.11: Nylon 4,6 production. Adapted from [211].
In the first step of polymerization, a prepolymer is prepared as a slurry in water. The amount of water added is preferably limited to 20wt%, whereby the temperature is kept between 190 and 220°C, usually at 210 °C. The use of a small amount of excess diamine has a positive influence on the properties of the polymer obtained after solid-phase polymerization. The excess ranges from 0.2 to 6 wt%, depending on the desired properties. The reaction is carried out in a closed vessel, and the reactor is operating under the elevated autogenous pressure, which is generally between 8 and 15 bar. Reaction time is as short as 30 min, depending on the conditions applied. Although the degree of polymerization in this stage is very low, between 6 and 14, this is the most advantageous prepolymer for the polycondensation. The latter process can take place at relatively moderate conditions and still produce a high molecular weight polyamide which is very stable. Discharging the reaction mixture from the reactor under a nitrogen atmosphere terminates the prepolymerization. The water present in the mixture is flashed off by slowly reducing the pressure to atmospheric. The discharge can also take place by spray-dying techniques or other similar processes. These have the significant advantage that the prepolymer can be readily obtained in powdered form. If spray drying is applied, the drying agent is hot nitrogen, which is largely recycled after drying and purification.
The postcondensation reaction takes place in the solid phase, usually in a fluidized bed at temperatures of 225-275 °C. The bed is kept in an inert (nitrogen) atmosphere with a water content of 20-50 vol% at almost atmospheric pressure. This step will take 4 h at most, which is relatively short. In order to obtain a uniform molecular weight distribution, it is important that the feed to the fluidized bed consists of particles with a uniform diameter. This is important for almost all polymers, but especially for Nylon 4,6 because it is not kept in the melt long enough for transamidation to establish a uniform molecular weight distribution. The excess diamine is removed from the nitrogen stream and recycled. The high molecular weight pellets leave the solid phase reactor and are cooled and stored under nitrogen to prevent degradation. The diaminobutane that results from the postpolymerization is removed by hot nitrogen, condensed after dust removal, and disposed. After the postcondensation, the nylon is led to an extruder, pelletized. and quenched with water. To remove the water, the slurry passes a preseparator and a dewatering screen. The pellets are then led to a fluidized bed dryer, where water is removed by hot air. The product is obtained at an overflow at the top of the fluidized bed.
B.5 Saturated and unsaturated polyesters
The well-known soda bottle is made of PET(poly-ethylene-terephtalate). This is only one of the many applications in which polyester is used. Others include clothing, audio— and videotapes, and some packaging applications. Polyesters are a class of polymers characterized by the so-called ester bond. A very important difference in the properties of saturated and unsaturated polyesters is their behavior at elevated temperatures. Saturated polyesters are thermoplastics, i.e., melt at certain temperatures, whereas unsaturated polyesters are thermoharders. Unsaturated polyesters form cross-links at higher temperatures, forming a network and thus hardening the polyester.
B.5.1 Saturated polyesters
The most produced saturated polyesters are PET (poly-ethylene-terephtalate) and PBT (poly-butyleneterephtalate); both are illustrated in Scheme B.3.
![]()
Scheme B.3: Mainstructure of PET (n = 2) and PBT (n = 4).
Both polyesters are produced in a similar batch process, which will be discussed in this chapter, with some minor differences. Still, the properties of PET and PBT are quite different, so some attention is paid to the relation between structure and properties. Production of polyesters can be done in two ways, either by transesterification or by direct esterification.
Transesterification
First, the process for PET production is described, and then the differences with PBT production are discussed. The typical layout of a batch process for the production of polyesters is given in Fig. B.12. The production process can be divided into three steps: melting, transesterification, and polycondensation. Dimethyl terephtalate (DMT) is melted in a stirred tank at 150—160°C under a nitrogen atmosphere; heating takes place by steam, carrier oil, or electricity. The molten DMT and ethylene glycol are reacted in heated and stirred transesterification reactors at 150-200 °C, operated at normal pressure in nitrogen atmosphere. At the start of the reaction, a lower temperature is favored to minimize the sublimation of DMT. The methanol which is formed during the reaction is continuously distilled off from the reaction mixture. Normally a glycol excess of 0.5-1 mol per mol of DMT is used to control the end groups and molecular weight. To achieve reasonable reaction rates, catalysts are necessary. Although catalyst listed in patent literature cover practically the whole periodic table, weak basic compounds, such as amines, oxides, alkoxides and acetates, are used as transesterification catalysts. Ca, Mg, Zn, Cd, Pb, and Co are reported to be the most effective. The transesterification product is added to the polycondensation reactor, which has to be equipped with a very efficient stirrer. Temperature is slowly increased to 250 °C, in the meantime decreasing the pressure. This way, the excess glycol is distilled off, and polycondensation can take place. Sufficiently high molecular weights can be obtained at temperatures of 270—280°C and a pressure lower than 1 mbar. In principle the transesterification catalyst can also be used here, but the properties of the product obtained are not satisfactory. Therefore the transesterification catalyst is masked with a phosphorous compound and replaced by a polycondensation catalyst, such as antimony, germanium, titanium, or lead compounds. Polycondensation is stopped when a predefined melt viscosity (and thus a defined molecular weight) is reached. The power consumption of the stirrer motor is often used as an indication for this, as it will increase with increasing melt viscosity. Removing the vacuum with oxygen-free nitrogen stops the polycondensation. This is done to prevent degradation of the polymer by oxidation. The melt is directly quenched with water and comminuted into chips or pellets. Before further processing the PET must be carefully dried at 80-130 °C to reduce the water content to below 0.01 wt%. During the drying process crystallization of the PET occurs, which is necessary in order to prevent agglomeration of the particles during prolonged storage. The comminuted PET is often subjected to solid-phase postcondensation for a fairly longtime (up to 20 h) at temperatures of up to 250 °C in an inert gas stream, resulting in an increase in molecular weight.
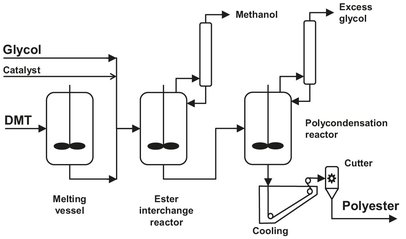
Fig. B.12: Batch process for polyester production. Adapted from [212].
Direct esterification
The direct esterification of terephtalic acid with ethylene glycol became important when economic processes were developed for producing fiber-grade acid. In new plants the direct esterification is the preferred method because of the higher reaction rate, and because higher molecular masses can be obtained and no transesterification catalyst is needed. The process is almost identical to the process for powder coating production, which will discussed later. The main difference in the process is the addition of organic solvent after part of the water is removed. This is a so-called solvatation step, in which bubbles are formed and the partial pressure of water is lowered, thus promoting the evaporation of the remainder of the water from the reaction mixture. The vapor is cooled and separated, after which the solvent is returned and the water disposed. Saturated polyesters can be produced at lower pressures than powder coatings, due to the composition of the reaction mixture.
Differences between PET and PBT production
As stated earlier, the production of PBT and PET are very similar. They differ only in raw material, catalyst, and some operating conditions. For PBT production transesterification is still preferred, whereas direct esterification is gaining popularity in the production of PET. For both products DMT is used as a raw material, but the comonomer for PBT is 1,4-butanediol instead of glycol. A significant byproduct in the production of PBT is tetrahydrofuran, which is formed from the diol. In PET production loss due to side-product formation is less than in its PBT counterpart. In PBT production, only one catalyst is added, which is used in transesterification and in polycondensation. Usually a tetra-alkyl titanate is used as a catalyst, because it is both very active and soluble.
B.5.2 Unsaturated polyesters
Usually, unsaturated polyesters (UP) are produced by batchwise condensation of di-carboxylic acids or anhydrides with diols. An often used, polyester is made of maleic anhydride, styrene, and diols. Even today the greater part of the UP resin is used in the production of glass fiber reinforced plastic. Dicarboxylic acids are esterified with diols at 180-230 °C in a nitrogen atmosphere in heatable and coolable reactors while stirring (see Fig. B.13). The capacity of the stainless steel reactors used ranges from 8 to 20 m2. Esterification is generally performed with a slight excess (up to 10 %) of diols. Usually the maleic anhydride is measured and added in the liquid state. The water formed in the reaction is distilled off. This is a necessary step because of the use of volatile glycols, which are separated from the water in the distillation column and recycled to the reactor. This is done in almost all polyester production processes.
Passage of nitrogen through the reaction mixture promotes removal of water and increases the esterification rate considerably. The end point of the condensation is determined by measuring the acid number and, possibly, also the hydroxyl number and the viscosity. Commercial UP resins have a mean molecular mass of 2000-4000. The liquid melt is cooled and then dissolved while stirring in styrene in coolable mixers. The styrene contains inhibitors such as hydroquinone or tertbutylcatechol. Also a catalyst is added, often a tin salt such as hydrated mono-butyl tin oxide. This catalyst is preferred because of the resulting product stability during storage. The esterification is reversible, and addition of steam will lower the degree of polymerization. This has the advantage that fine tuning of the molecular weight can be easily done. Even if the same raw materials are used, the resin products may differ as regards their processing properties and end properties, depending on the amount of excess glycol, the temperature, and reaction conditions. In the case of raw materials with widely differing esterification rates, two-stage condensation must be used to obtain uniform buildup of the polyester chain and thus favorable end properties. Azeotropic esterification is important only for certain paint resins. An entraining agent (e.g., toluene or xylene) is added, and during condensation the water is distilled off together with the toluene. The toluene is then separated from the water with a dephlegmator and returned to the reactor. At the end of the reaction the toluene must be removed from the resin (e.g., with a thin-film evaporator).
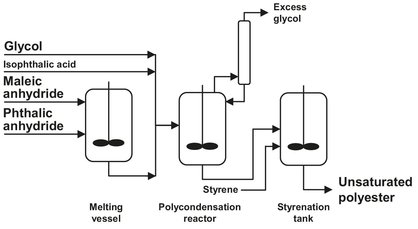
Fig. B.13: Batchwise production of UP resins. Adapted from [212].
B.5.3 Powder coatings
A special application of polyester resins is in powder coatings. These are used for protection of underlying materials, decoration and modification of the surface properties. A powder coating mainly consists of a (fine) polyester powder, to which several substances are added to obtain the necessary properties. Examples of these substances are coloring agents, hardeners, stabilizers, and sometimes paraffin. Powder coatings can be applied in several ways; by a fluid bed process, by flaking, or by spraying.
Production of powder coatings is usually done batchwise, although very large batches are needed to produce a constant-quality powder. After the initial production, it is hard to modify the properties of the coating, so all process parameters have to be kept constant. Large batches are also needed, because cleaning of the equipment is time consuming and expensive. Cleaning has to be done very thoroughly, because it might be possible that two coatings are incompatible. The applied batch reactors are designed for a wide pressure range (vacuum to several bar), and equipped with internal heating/cooling coils and a multitask agitator. First the glycols, acids, and catalyst are added to the reactor, which is then closed and heated at high speed. Due to the poor solubility of the acids in glycol, the reactor content is a slurry. This results in rising pressure and a slow-starting reaction. When a predefined pressure level is reached, a valve to a distillation column is opened. In this column, separation of water and glycol takes place, water is disposed of, and the glycol is recycled. Due to the evaporation of water, the temperature rise is slower. The pressure is kept at a constant level to ensure good operation of the distillation column, e.g. to avoid flooding. Another reason to keep the pressure at an elevated level is the formation of oligomers in the reactor, thus reducing the amount of volatiles. When the temperature reaches a value dictated by the desired product specification, pressure is slowly reduced. This is possible because the amount of volatiles has decreased enough for safe operation of the distillation column. When atmospheric pressure is reached, it is important to remove the water as fast as possible to preserve the conversion obtained. If the water would not be removed, the polyester would slowly degrade because of the equilibrium reaction. Most of the water is removed at atmospheric pressure, and the remainder is removed at reduced pressure. After cooling the reactor contents to a suitable temperature, the desired additives can be added. After mixing, the polymer is sent to a cooling belt, where further cooling to room temperature takes place and flakes are formed.